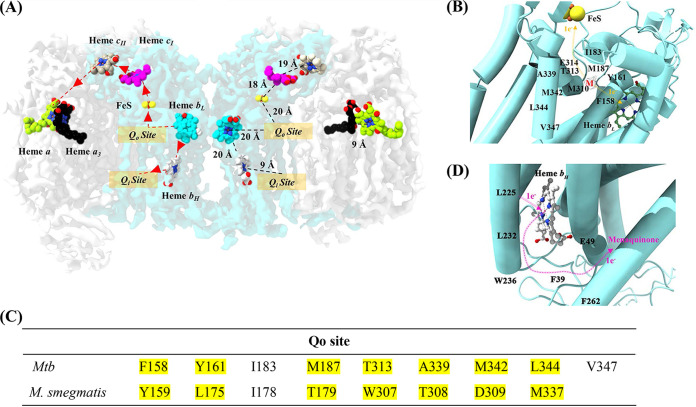
FIG 5.
Electron transfer in resolved M. tuberculosis cyt-bcc:aa3. (A) Electron transfer pathway. The left side of the dimer describes the electron transfer pathway, while the right side depicts the distances between the reaction centers. The binding of menaquinol to the Qo site in QcrB initiates the electron transfer process. The first electron from menaquinol is transferred to FeS, which is ~20 Å from the Qo site. The second electron from menaquinol is transferred to heme bL, which is ~20 Å apart from the Qo site and subsequently transferred to heme bH. The electron from heme bH is used to reduce a menaquinone bound at the Qo site. FeS transfers its electron to the cytochrome c domain present in QcrC, which transfers the electron further to the cyt-aa3 domain. The electrons are carried in the following order: from FeS to cyt-cI, cyt-cII, and CuA. The final electron transfer pathway occurs from CuA to heme a followed by CuB and heme a3. The electrons at heme a3 are used to reduce O2 to H2O. (B) Electron transfer in the Qo site. The Qo binding site with side chains interacting with menaquinol (M). The electron transfer pathway is illustrated with orange arrows. The binding of menaquinol results in the transfer of one electron to heme bL and the other to FeS. (C) Amino acid residues that interact with menaquinol at the Qo site revealed in the M. tuberculosis cyt-bcc:aa3 and the M. smegmatis cyt-bcc:aa3 structure. (D) Electron transfer in the QN site. The Qi binding site with side chains is predicted to interact with menaquinone. The electron transfer pathway is illustrated by pink arrows. The electron, which originated from heme bL, is transferred to heme bH before being transferred to menaquinone in the Qi site. The [2Fe-2S] and heme groups are shown as balls and sticks and are labeled in the figures. Mtb, M. tuberculosis.