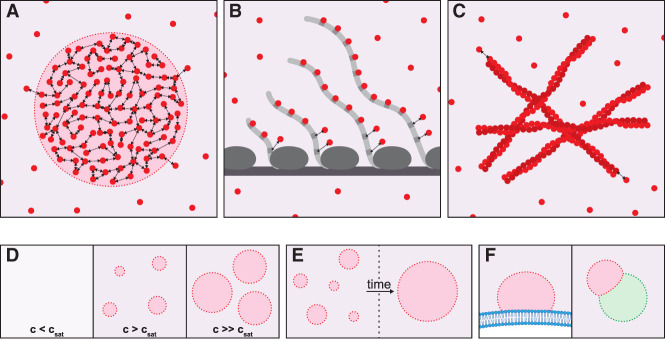
Figure 1.
Condensates and other assemblies. (A) Condensates arise when diffusive multivalent molecules (red circles) interact reversibly (double arrows) to form a dynamic network. Condensates are defined by an interface (red dotted line), with associated surface tension separating the condensed phase (red) from the dilute phase (purple). The surface tension arises from the energy differential between molecules at the interface (which are pulled into the condensate by their neighbors) and molecules in the interior. The molecules inside the condensate experience a chemical and diffusive environment distinct from the dilute phase. (B) A multivalent scaffold (such as nascent RNA molecules) can concentrate proteins (red) that bind to the scaffold (gray). Such an assembly may resemble a condensate by microscopy but does not possess an interface and therefore is not phase-separated. However, this type of assembly could evolve into a condensate if the proteins, in addition to binding to the scaffold, also interact with one another and binding to the scaffold causes the proteins to exceed csat locally. (C) Proteins containing low-complexity, prion-like domains can interact via β-sheet stacking to form extended fibers in multiple dimensions. This type of assembly does not constitute a phase-separated condensate but could arise within a condensate that concentrates proteins with prion-like domains. (D–F) Properties of condensates. (D) Condensates form above csat (saturation concentration), the maximum concentration allowed in the dilute phase. Above csat, further increases in concentration cause the condensates to grow larger without any changes to the concentration in the dilute phase, which remains at csat. However, this theoretical prediction is difficult to apply in vivo, where multiple components contribute to csat, leading to complex concentration-dependent behaviors (Riback et al. 2020). (E) Surface tension drives condensates to minimize surface area, causing them to coarsen over time to create fewer, larger condensates with lower surface:volume ratios. The time scale of coarsening will depend on the material properties of the condensates (less dynamic condensates will coarsen more slowly). Also, agents that adsorb to the interface can reduce surface tension and coarsening. (F) Condensates wet surfaces, including membranes (blue) and other condensate types (green) that provide favorable interaction interfaces (Gouveia et al. 2022). For example, P granules wet nuclear membranes, and P-bodies wet stress granules.