Fig. 4.
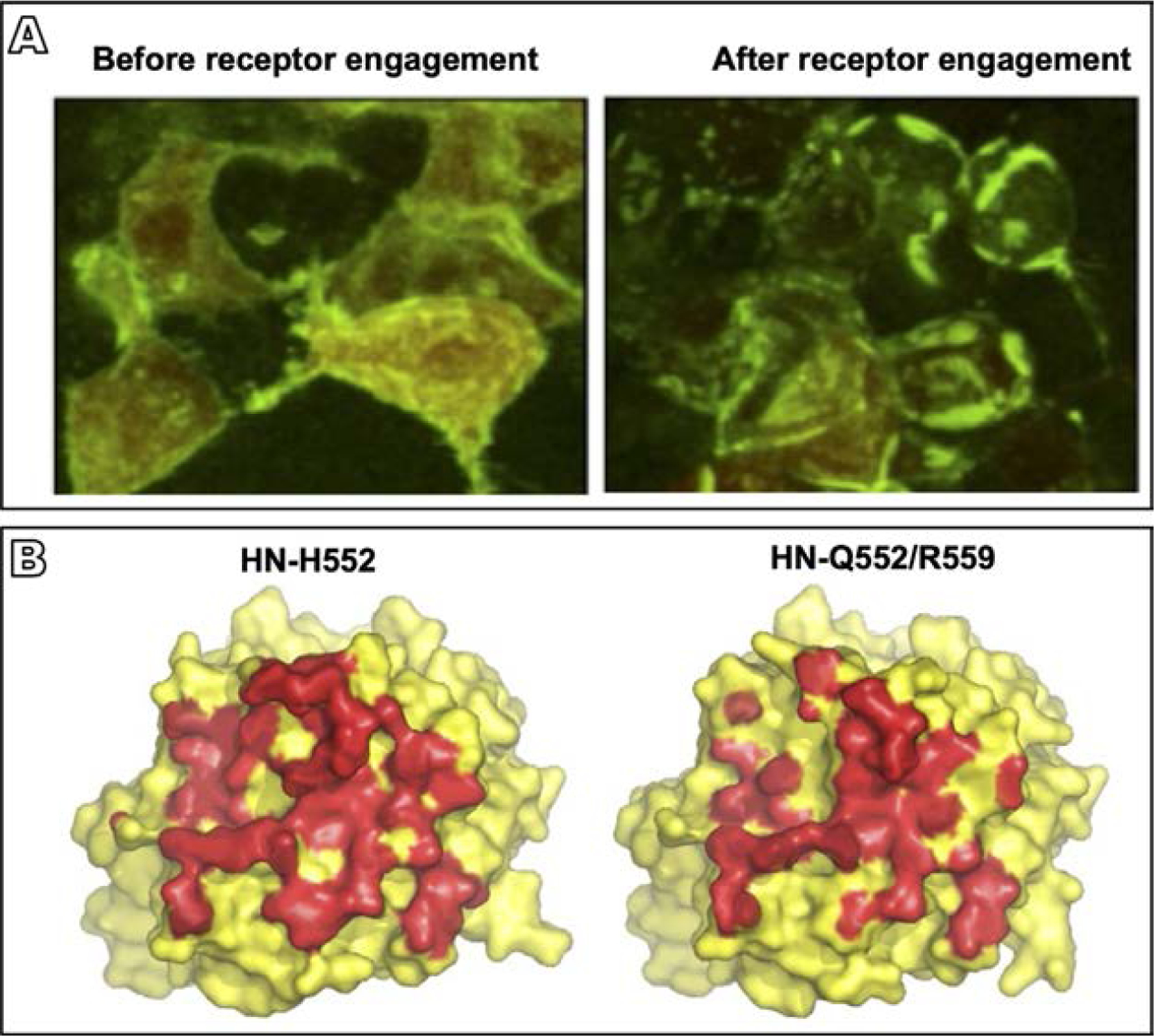
HPIV3 HN-F interaction and the role of the HN dimer interface in this process. (A) 293T cells co-transfected with constructs encoding HN and F, each with their corresponding bimolecular fluorescence complementation tag, were treated overnight with zanamivir to prevent HN-receptor binding (Porotto et al., 2012a). Evenly distributed fluorescence was visualized across the cell surface in the absence of receptor engagement (left panel), but after the zanamivir was removed and receptor engagement was permitted, HN-F interaction occurred in clusters at the sites of cell-cell contact (right panel) (B) Surface rendering of the crystal structure of the HN monomer globular head viewed from above for HN with the indicated mutated site II residues (Xu et al., 2013). HN residues in contact with the opposing monomer in the dimer interface are shown in red. Introduction of the double mutation Q552/R559 in site II results in the loss of about 800 of 3700Å2 buried interface area in the dimer (right hand side), revealing the effect of alterations in this site to the dimer structure. Adapted from Porotto, M., Palmer, S.G., Palermo, L.M., Moscona, A., 2012a. Mechanism of fusion triggering by human parainfluenza virus type III: communication between viral glycoproteins during entry. J. Biol. Chem. 287 (1), 778–93, with permission; Xu, R., Palmer, S.G., Porotto, M., Palermo, L.M., Niewiesk, S., Wilson, I.A., et al., 2013. Interaction between the hemagglutinin-neuraminidase and fusion glycoproteins of human parainfluenza virus type III regulates viral growth in vivo, MBio. 4 (5), e00803–13, with permission.
