Abstract
Objective
To characterize energy and macronutrient intakes in Brazil and to describe the top food items contributing to energy and macronutrient intakes.
Design
Two non-consecutive 24 h dietary records were collected and energy and macronutrient data were adjusted for usual intake distribution. Descriptive statistics and ANOVA with the Bonferroni post hoc test were analysed using SAS version 9·1. Means and standard deviations were estimated for sex, age and income strata.
Setting
Nationwide cross-sectional survey, 2008–2009.
Subjects
Nationally representative sample of individuals ≥10 years old (n32 749), excluding pregnant and lactating women (n 1254).
Results
The average energy intake was 7958 kJ/d (1902 kcal/d) and mean energy density was 6·82 kJ/g (1·63 kcal/g). Added sugar represented 13 % of total energy intake and animal protein represented 10 %. The mean contribution of total fat to energy intake was 27 %, while the mean saturated fat contribution was 9 %. Compared with the lowest quartile of income, individuals in the highest income quartile had greater mean intakes of energy, added sugar, alcohol, animal protein, total fat, saturated fat, monounsaturated fat and trans fat. Rice, beans, beef, bread and coffee were among the top five foods contributing most to the intakes of energy, carbohydrates, protein, fat and fibre.
Conclusions
In general, Brazilians’ dietary intake is compatible with a high risk of obesity and non-communicable chronic diseases, being characterized by high intakes of added sugar and saturated fat. Income may be a major determinant of diet nutritional characteristics.
Keywords: Food consumption, Dietary survey, Food records, Energy intake
Similar to many other countries, the dietary patterns in Brazil have changed rapidly and drastically in recent decades. These changes are characterized by the increased intake of processed foods and consequently of saturated fats and simple sugars. The excessive consumption of these foods has been linked to the development of excessive weight gain and to an increased risk CVD( 1 ) as obesity is a leading risk factor for many adverse health outcomes including dyslipidaemia, hypertension, type 2 diabetes, CVD and certain types of cancer( 2 ). In 2007, 72 % of deaths in Brazil were attributed to non-communicable diseases, especially stroke, CVD and cancer( 3 ).
Additionally, the prevalence of overweight and obesity in Brazil has increased steadily since 1974 in both females and males, as well as across all income quintiles. In the 35 years elapsed from 1974–1975 to 2008–2009, the prevalence of overweight in adults almost tripled among males (18·5 % to 50·1 %) and almost doubled among females (from 28·7 % to 48·0 %). In the same period, the prevalence of obesity more than quadrupled among males (from 2·8 % to 12·4 %) and doubled among females (from 8·0 % to 16·9 %)( 4 ).
Despite the importance of diet in the development of these diseases, which are among the main causes of mortality in Brazil( 3 ), and the accelerated progression of overweight and obesity( 4 ), Brazil had not collected information about food consumption at the individual level until a few years ago. Food consumption trends were based on information from the Household Expenditure Surveys (HES), which have been conducted regularly since the mid-1970s.
According to data from the two most recent HES (2002–2003 and 2008–2009), household macronutrient availability in Brazil has changed, with reductions in total carbohydrate content and concomitant increases in protein and fat contents( 5 , 6 ). In both studies, the energy intake from added sugar exceeded the maximum level of 10 % of total energy intake proposed by the WHO( 7 ); additionally, the increase in protein intake was attributable to that from animal sources rather than from vegetable sources, whereas among fats increased intake of both saturated (from 7·9 % to 8·3 % of total energy intake) and monounsaturated fat (from 8·7 % to 9·2 % of total energy intake) was observed.
In Brazil, current trends in food habits have indicated significant increases in away-from-home food consumption since expenditures on away-from-home food consumption increased from 24·1 % (2002–2003) to 31·1 % (2008–2009) of total food expenditures. During the same period, the household energy availability decreased from 7531 kJ to 6694 kJ (1800 kcal to 1600 kcal)( 6 ). These tendencies suggest that the HES may not be a consistent source of individual dietary intake data.
Therefore, the Brazilian government has made efforts to collect data of individual food consumption through the development of the first Brazilian Individual Dietary Survey along with the 2008–2009 HES, which was conducted by the Instituto Brasileiro de Geografia e Estatística (Brazilian Office of Geography and Statistics). This survey collected information about food intake from a nationally representative sample of individuals ≥10 years old( 8 ). Such data could provide important tools for the planning and monitoring of health and nutrition activities. For that reason, the purpose of the present study was to characterize the energy and macronutrient intakes in Brazil and to describe the food items that contributed most to these intakes.
Methods
Participants
Data were collected in a nationally representative cross-sectional survey, the 2008–2009 Brazilian HES, which investigated a sample of 55 970 households that had been selected using a two-stage cluster sample design( 9 ). In the first stage, census tracts, which were the primary sampling units, were randomly selected according to the 2000 Brazilian Demographic Census in order to obtain homogeneous socio-economic and geographic strata. In the second stage, households were selected within each tract by simple random sampling without replacement. The National Dietary Survey was conducted in about 24 % of these households (n13 569) to obtain food consumption data for all family members ≥10 years old. The present analysis included 32 749 individuals, after excluding 1254 pregnant and lactating women.
Dietary intake
All eligible individuals were asked to describe all foods and beverages consumed during two specified non-consecutive days in order to complete the food records. Participants were asked to record the amounts consumed and the times and places where the foods were consumed; additionally, information about cooking methods was required for certain items (primarily meats and vegetables). Water intake was not recorded. The respondents received instruction manuals, with photographs of common household measures, explaining how to complete the records. Information was collected during the period between waking up on the specified day and before waking up on the following day.
All dietary records were reviewed by the interviewers, who probed the respondents for commonly forgotten foods and periods longer than 3 h without any reported intake. Additionally, food records with fewer than five items were checked to ensure that no additional items had been consumed during the day.
A compiled nutritional database( 10 ), based mainly on the Brazilian Food Composition Table( 11 ) and the Nutrition Coordination Center Nutrient Databank( 12 ), was used to estimate the daily energy and macronutrient intakes. The relative contribution of each macronutrient to total energy intake was also calculated. Energy density was defined as the amount of energy available in a given weight of food (kJ/kcal per 100 g) and only solid foods (except for beverages) were considered in the calculation( 13 ). Additionally, information on the consumption of sugar and/or artificial sweetener was collected using a specific question: ‘What do you use more frequently: sugar, artificial sweetener, both, or none?’ The amount of table sugar added to beverages (except mate * and flavoured drink mix) was computed using standardized procedures defined by the Instituto Brasileiro de Geografia e Estatística( 14 ): if the respondent informed that ‘sugar is frequently used in beverages’, then 10 % of sugarcane was added to the beverage (10 g of sugar for each 100 ml of beverage); if the respondent informed to use both sugar and artificial sweetener, then 5 % of sugarcane was added to the beverage (5 g of sugar for each 100 ml of beverage). The amount of added sugar from processed foods was computed using the Nutrition Coordination Center Nutrient Databank.
To adjust for usual intake distribution, we estimated within-individual variations derived from two days of food records using the Multiple Source Method (https://msm.dife.de). The Multiple Source Method is characterized by a two-part shrinkage technique applied to the residuals of two regression models: (i) one for positive daily intake data; and (ii) one for event of consumption( 15 ). Finally, the contribution (%) of food groups to energy and macronutrient intakes was estimated and the top five food groups contributing to the dietary intake were described.
Statistical analyses
Means and standard deviations of energy and macronutrient intakes were estimated for each age-and-sex stratum and according to per capita monthly household income quartiles. Age was classified into four categories: (i) 10–13 years (adolescents – first phase of adolescence); (ii) 14–18 years (adolescents – second phase of adolescence); (iii) 20–59 years (adults); and (iv) ≥60 years (elderly). Per capita monthly household income was calculated as the total monthly household income divided by the number of individuals in the household (this included both monetary and non-monetary sources of income, including donations, gifts and self-production) and was divided into quartiles as follows: quartile 1, <95 dollars; quartile 2, 95–178 dollars; quartile 3, 179–332 dollars; and quartile 4, >332 dollars.
Differences in the mean values were assessed with one-way ANOVA with the Bonferroni post hoc test. All statistical analyses were performed considering the sampling design and weights using the statistical software package SAS version 9·1.
Results
Energy and macronutrient intakes
Overall, the mean daily energy intake was 7958 kJ (1902 kcal) and the mean energy density, considering only solid food, was 6·82 kJ/g (1·63 kcal/g). The mean carbohydrate contribution to total energy intake was 56 % and the mean contribution of added sugar to total energy intake was 13 %. Protein provided 17 % of total energy intake, with animal protein corresponding to 10 % of total energy intake. The mean contribution of total fat to total energy intake was 27 %; saturated fat 9 %, polyunsaturated fat 6 %, monounsaturated fat 9 % and trans-fat 1·1 %. The mean daily intakes of linoleic and linolenic acids were 11 and 1·4 g, respectively, and the mean cholesterol intake was 253 mg/d. The mean fibre intake was 20 g/d, of which 11 g/d was accounted for by insoluble fibre. Finally, the mean contribution of alcohol to total energy intake was 0·4 % (Table 1).
Table 1.
Dietary guidelines, and mean daily intakes of energy (kilojoules/kilocalories) and macronutrients (grams and percentage contribution to total energy intake); National Dietary Survey, Brazil, 2008–2009
| Brazil (n 32 749) | |||
|---|---|---|---|
| Energy and macronutrients | Dietary guidelines | Mean | sd |
| Total energy (kJ) | – | 7958 | 2753 |
| Total energy (kcal) | – | 1902 | 658 |
| Energy density (kJ/g) | 5·23 kJ/g | 6·82 | 1·09 |
| Energy density (kcal/g) | <1·25 kcal/g( 13 ) | 1·63 | 0·26 |
| Total carbohydrates (g) | – | 263 | 96 |
| % of total energy intake | 55–75 % of total energy intake( 7 ) | 56 | 7 |
| Added sugar (g) | – | 64 | 40 |
| % of total energy intake | <10 % of total energy intake( 7 ) | 13 | 6 |
| Total fibre (g) | >25 g/d( 2 ) | 20 | 9 |
| Insoluble fibre (g) | – | 11 | 6 |
| Total protein (g) | – | 80 | 32 |
| % of total energy intake | 10–15 % of total energy intake( 7 ) | 17 | 3 |
| Animal protein (g) | – | 50 | 26 |
| % of total energy intake | – | 10 | 4 |
| Total fat (g) | – | 58 | 23 |
| % of total energy intake | 15–30 % of total energy intake( 7 ) | 27 | 5 |
| Saturated fat (g) | – | 20 | 9 |
| % of total energy intake | <7 % of total energy intake( 18 ) | 9 | 2 |
| Polyunsaturated fat (g) | – | 13 | 5 |
| % of total energy intake | <10 % of total energy intake( 17 ) | 6 | 1 |
| Monounsaturated fat (g) | – | 19 | 8 |
| % total energy intake | – | 9 | 2 |
| Trans fat (g) | 2·4 | 1·8 | |
| % of total energy intake | <1 % of total energy intake( 7 ) | 1·1 | 0·7 |
| Cholesterol (mg) | <300 mg/d( 7 ) | 253 | 127 |
| Linoleic acid (n-6) (g) | – | 11 | 4·3 |
| Linolenic acid (n-3) (g) | – | 1·4 | 0·6 |
| n-6:n-3 ratio | 4:1 to 5:1( 16 ) | 7·6 | 1·0 |
| Alcohol (g) | – | 1·5 | 9·1 |
| % of total energy intake | – | 0·4 | 2·3 |
Table 1 also presents internationally accepted dietary recommendations that were used for comparison with the intakes of selected macronutrients in Brazil( 7 , 13 , 16 – 18 ). Overall, the mean percentage contributions of added sugar, saturated fat and trans-fat to total energy intake were above the recommended levels, while the mean percentage contributions of carbohydrates and total fat were in agreement with the guidelines. However, the mean daily intake of fibre (recommendation=25 g/d)( 7 ) and the mean percentage contribution of polyunsaturated fat to total energy intake (recommendation<10 %)( 17 ) remained below the recommended levels.
Energy and nutrient intakes by sex and age
In the total sample, 9·4 % of the participants were between 10 and 13 years old, 11·3 % were between 14 and 18 years old, 66·1 % were adults (19–59 years old) and 13·2 % were elderly (≥60 years old). Males generally reported a higher level of total energy intake than did females; this difference was more pronounced among adults as the daily energy intake of adult men was approximately 25 % higher than that of adult women (P<0·01). There was no significant difference in the daily energy intakes of 10–13-year-old males and females; however, the daily energy intake of 14–18-year-old males was 15 % higher than that of females in the same age group (P<0·01). Among the elderly, the difference in mean daily energy intakes between men and women was about 18 % (P<0·01; Tables 2 and 3).
Table 2.
Mean daily intakes of energy (kilojoules/kilocalories) and macronutrients (grams and percentage contribution to total energy intake) of males according to age group; National Dietary Survey, Brazil, 2008–2009
| 10–13 years | 14–18 years | 19–59 years | ≥60 years | |||||
|---|---|---|---|---|---|---|---|---|
| (n 1515) | (n 1905) | (n 10 287) | (n 1993) | |||||
| Energy and macronutrients | Mean | sd | Mean | sd | Mean | sd | Mean | sd |
| Total energy (kJ) | 8130a | 2757 | 9113b | 3167 | 8895b | 2962 | 7648c | 2548 |
| Total energy (kcal) | 1943a | 659 | 2178b | 757 | 2126b | 708 | 1828c | 609 |
| Energy density (kJ/g) | 7·11a | 0·92 | 7·20a | 0·88 | 6·82b | 0·88 | 6·40c | 0·92 |
| Energy density (kcal/g) | 1·70a | 0·22 | 1·72a | 0·21 | 1·63b | 0·21 | 1·53c | 0·22 |
| Total carbohydrates (g) | 274 | 98 | 303 | 110 | 289 | 102 | 251 | 91 |
| % of total energy intake | 57a | 7 | 56b | 7 | 55c | 7 | 56b,c | 7 |
| Added sugar (g) | 70 | 41 | 77 | 47 | 67 | 41 | 52 | 34 |
| % of total energy intake | 14a | 6 | 14a | 6 | 13b | 6 | 11c | 6 |
| Total fibre (g) | 20a | 9 | 22b | 9 | 23b | 10 | 21c | 10 |
| Insoluble fibre (g) | 11a | 6 | 12b,c | 7 | 13b | 7 | 12a,c | 6 |
| Total protein (g) | 78 | 30 | 88 | 35 | 91 | 35 | 81 | 32 |
| % of total energy intake | 16a | 3 | 17b | 3 | 17c | 3 | 18d | 3 |
| Animal protein (g) | 47 | 26 | 53 | 29 | 56 | 30 | 51 | 27 |
| % of total energy intake | 10a | 4 | 10a | 4 | 11b | 4 | 11b | 4 |
| Total fat (g) | 60 | 24 | 67 | 27 | 64 | 26 | 55 | 21 |
| % of total energy intake | 27a | 5 | 27a | 5 | 27a | 5 | 26b | 5 |
| Saturated fat (g) | 21 | 10 | 23 | 11 | 22 | 10 | 19 | 8 |
| % of total energy intake | 9a | 2 | 9a | 2 | 9b | 2 | 9b | 2 |
| Polyunsaturated fat (g) | 13 | 5 | 14 | 6 | 14 | 5 | 12 | 5 |
| % of total energy intake | 6a,b | 1 | 6a,c,d | 1 | 6c | 1 | 6b,d | 1 |
| Monounsaturated fat (g) | 20 | 9 | 23 | 10 | 21 | 9 | 18 | 8 |
| % of total energy intake | 9a | 2·0 | 9a | 2·0 | 9a | 2·0 | 9b | 2·0 |
| Trans fat (g) | 2·3 | 1·7 | 2·8 | 2·1 | 2·5 | 2·0 | 2·2 | 1·7 |
| % of total energy intake | 1·10a | 0·7 | 1·13b | 0·8 | 1·10a | 0·7 | 1·00a | 0·7 |
| Cholesterol (mg) | 252a | 125 | 279b | 142 | 280b | 141 | 248a | 129 |
| Linoleic acid (n-6) (g) | 11·0a | 4·2 | 12·4b | 4·8 | 12·1b | 4·6 | 10·3c | 4·0 |
| Linolenic acid (n-3) (g) | 1·4a | 0·5 | 1·6b | 0·6 | 1·6b | 0·6 | 1·4a | 0·5 |
| n-6:n-3 ratio | 7·8a | 1·1 | 7·8a | 1·0 | 7·7b | 0·9 | 7·6c | 1·0 |
| Alcohol (g) | 0·08 | 2·5 | 0·3 | 4·4 | 3·3 | 14·4 | 2·3 | 7·7 |
| % of total energy intake | 0·03a | 0·83 | 0·09a | 1·60 | 0·83b | 3·40 | 0·69b | 2·30 |
a,b,c,dMean values within a row with unlike superscript letters were significantly different (P < 0·05).
Table 3.
Mean daily intakes of energy (kilojoules/kilocalories) and macronutrients (grams and percentage contribution to total energy intake) of females according to age group; National Dietary Survey, Brazil, 2008–2009
| 10–13 years | 14–18 years | 19–59 years | ≥60 years | |||||
|---|---|---|---|---|---|---|---|---|
| (n 1566) | (n 1811) | (n 11 344) | (n 2328) | |||||
| Energy and macronutrients | Mean | sd | Mean | sd | Mean | sd | Mean | sd |
| Total energy (kJ) | 7816a | 2469 | 7945a | 2615 | 7201b | 2268 | 6498c | 2054 |
| Total energy (kJ) | 1868a | 590 | 1899a | 625 | 1721b | 542 | 1553c | 491 |
| Energy density (kJ/g) | 7·11a | 0·92 | 7·28b | 1·00 | 6·74c | 0·88 | 6·28d | 0·92 |
| Energy density (kcal/g) | 1·70a | 0·22 | 1·74b | 0·24 | 1·61c | 0·21 | 1·50d | 0·22 |
| Total carbohydrates (g) | 264 | 88 | 269 | 94 | 240 | 81 | 217 | 74 |
| % of total energy intake | 57a,b | 6 | 57a | 6 | 56c | 7 | 56b,c | 7 |
| Added sugar (g) | 73 | 41 | 78 | 45 | 61 | 36 | 46 | 30 |
| % of total energy intake | 15a | 7 | 16b | 7 | 14c | 6 | 12d | 6 |
| Total fibre (g) | 18 | 7 | 18 | 8 | 18 | 8 | 18 | 7 |
| Insoluble fibre (g) | 10 | 5 | 10 | 5 | 10 | 5 | 10 | 5 |
| Total protein (g) | 75 | 27 | 74 | 28 | 73 | 26 | 69 | 26 |
| % of total energy intake | 16a | 3 | 16b | 3 | 17c | 3 | 17d | 3 |
| Animal protein (g) | 47 | 23 | 46 | 23 | 47 | 22 | 44 | 22 |
| % of total energy intake | 10a | 4 | 10a | 4 | 11b | 4 | 11b | 4 |
| Total fat (g) | 58 | 22 | 59 | 23 | 53 | 20 | 48 | 18 |
| % of total energy intake | 27a,b | 5 | 27a | 5 | 27b | 5 | 27c | 5 |
| Saturated fat (g) | 20 | 9 | 21 | 9 | 19 | 8 | 17 | 7 |
| % of total energy intake | 10 | 2 | 10 | 2 | 9 | 2 | 9 | 2 |
| Polyunsaturated fat (g) | 12 | 5 | 12 | 5 | 11 | 4 | 10 | 4 |
| % of total energy intake | 6a,b | 1 | 6a | 1 | 6a | 1 | 6b | 1 |
| Monounsaturated fat (g) | 19 | 8 | 20 | 8 | 18 | 7 | 16 | 6 |
| % of total energy intake | 9a | 2 | 9a | 2 | 9a | 2 | 9b | 2 |
| Trans fat (g) | 2·2 | 1·6 | 2·4 | 1·8 | 2·2 | 1·6 | 2·0 | 1·5 |
| % of total energy intake | 1·1a | 0·7 | 1·1a,b | 0·7 | 1·1b | 0·7 | 1·1a,b | 0·7 |
| Cholesterol (mg) | 243a,b | 112 | 244a | 116 | 234b | 109 | 215c | 104 |
| Linoleic acid (n-6) (g) | 10·4a | 4·1 | 10·8a | 4·3 | 9·7b | 3·6 | 8·6c | 3·3 |
| Linolenic acid (n-3) (g) | 1·3a | 0·5 | 1·4b | 0·5 | 1·3c | 0·5 | 1·2d | 0·4 |
| n-6:n-3 ratio | 7·9a | 1·0 | 7·8a | 1·3 | 7·7b | 1·1 | 7·6c | 1·0 |
| Alcohol (g) | 0·02 | 0·08 | 0·28 | 4·30 | 0·66 | 5·40 | 0·30 | 1·90 |
| % of total energy intake | 0·007a | 0·03 | 0·09b | 1·40 | 0·22c | 1·70 | 0·14b,c | 0·95 |
a,b,c,dMean values within a row with unlike superscript letters were significantly different (P < 0·05).
Energy intake varied according to age for both male and female individuals. Daily energy intake estimated for 10–13-year-old males was 12 % lower than that observed for 14–18-year-old males (P<0·01). In contrast, no difference in energy intake was observed for females in the same age groups. When adolescents and adults were compared, differences in energy intake were observed only for females, with adult women reporting energy intake 10 % lower than that of female adolescents (P<0·01). The mean energy intake of elderly men (>60 years) was 14 % less than that estimated for adult men (P<0·01) and that estimated for elderly women was 10 % smaller than that observed for adult women (P<0·01). Elderly and adolescent participants had the lowest and the highest means of dietary energy density, respectively (Tables 2 and 3).
For both sexes, the percentage contribution of carbohydrates to total energy intake was higher for adolescents; however, these differences were significant only when 10–13-year-old males were compared with adult and elderly men (Tables 2 and 3).
For all age groups, females reported a higher percentage contribution of added sugar to energy intake than did males, particularly in the 14–18-year-old (16 % v. 14 %; P<0·01) and adult (14 % v. 13 %; P<0·01) age groups. For both males and females, the mean contribution of added sugar decreased with age (Tables 2 and 3).
The mean daily total fibre intake was related to total energy intake, being significantly higher in males compared with females, although among the latter, the mean daily fibre intake did not vary across age groups and was approximately 18 g.
As expected, the percentage contribution of alcohol to total energy intake was higher in males than in females, except for the 14–18-year-old group, in which the contribution was the same for both sexes. For both males (0·83 % v. 0·69 %) and females (0·22 % v. 0·14 %), the contribution of alcohol was higher in adults than in elderly individuals (Tables 2 and 3).
For both sexes, elderly participants reported a higher energy contribution from protein (18 % for men, 17 % for women) than adolescents and adults. Adults and elderly of both sexes reported a higher energy contribution from animal protein than adolescents. In both sexes, the elderly reported the lowest contribution of total fat to total energy intake (26 % for men and 27 % for women; Tables 2 and 3).
In comparison to adults and the elderly, adolescents of both sexes reported the highest percentage contribution of saturated fat to total energy intake, although these differences were significant only for adolescent females. Additionally, females generally reported a higher contribution of saturated fat to total energy intake than males in all age groups (Tables 2 and 3).
The lowest percentage contributions of polyunsaturated and monounsaturated fats to total energy intake were observed among the elderly; however, no significant differences were observed between males and females. The energy contribution of trans-fats was 1·1 % for nearly all sex-and-age strata, except in elderly men (1·0 %; Tables 2 and 3).
Males reported greater mean daily cholesterol intake than did females, except for the 10–13-year-old group, and the greatest difference (approximately 20 %) was observed when adult men were compared with adult women (280 v. 234 mg; P<0·01). The mean daily cholesterol intake decreased with age in both sexes. This decrease was more pronounced among males, although elderly women presented the lowest mean cholesterol intake (215 mg/d). There was no sex-related difference in the n-6:n-3 fatty acid ratio, although this ratio decreased with age in both sexes (Tables 2 and 3).
Energy and nutrient intakes according to income
Comparing mean energy intake across the quartiles of per capita monthly household income showed that the mean energy intake in quartile 4 (wealthiest) was 10 % higher than that estimated for quartile 1 (poorest). The same trend was observed for the mean contribution to total energy intake from added sugar (+12 %), alcohol (+700 %), animal protein (+8 %), total fat (+9 %), saturated fat (+19 %), monounsaturated fat (+10 %) and trans-fat (+22 %; Table 4). Mean contributions of total carbohydrates (−4 %) and polyunsaturated fat (−5 %) to energy intake and the n-6:n-3 ratio were lower in the quartile 4 compared with quartile 1 (Table 4).
Table 4.
Mean daily intakes of energy (kilojoules/kilocalories) and macronutrients (grams and percentage contribution to total energy intake) according to per capita monthly household income quartile; National Dietary Survey, Brazil, 2008–2009
| Per capita monthly household income quartiles | ||||||||
|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||||
| (n 8185) | (n 8189) | (n 8185) | (n 8190) | |||||
| Energy and macronutrients | Mean | sd | Mean | sd | Mean | sd | Mean | sd |
| Total energy (kJ) | 7481a | 2682 | 7971b | 2703 | 8004b | 2757 | 8201c | 2807 |
| Total energy (kcal) | 1788a | 641 | 1905b | 646 | 1913b | 659 | 1960c | 671 |
| Energy density (kJ/g) | 6·82 | 0·92 | 6·82 | 0·92 | 6·86 | 0·92 | 6·78 | 0·88 |
| Energy density (kcal/g) | 1·63 | 0·22 | 1·63 | 0·22 | 1·64 | 0·22 | 1·62 | 0·21 |
| Total carbohydrates (g) | 252 | 95 | 265 | 94 | 265 | 95 | 267 | 98 |
| % of total energy intake | 57a | 7 | 56b | 7 | 56b | 7 | 55c | 6 |
| Added sugar (g) | 54 | 33 | 63 | 39 | 65 | 41 | 70 | 43 |
| % of total energy intake | 12a | 6 | 13b | 6 | 13b,c | 6 | 14c | 6 |
| Total fibre (g) | 20a,b | 9 | 21a | 9 | 20a | 9 | 20b | 9 |
| Insoluble fibre (g) | 11a | 7 | 11b | 6 | 11b | 6 | 11a | 5 |
| Total protein (g) | 78 | 34 | 80 | 32 | 80 | 31 | 82 | 30 |
| % of total energy intake | 17a | 4 | 17b | 3 | 17b | 3 | 17a,b | 3 |
| Animal protein (g) | 47 | 29 | 49 | 26 | 50 | 25 | 54 | 24 |
| % of total energy intake | 10a | 5 | 10a | 4 | 10a | 4 | 11b | 3 |
| Total fat (g) | 52 | 22 | 58 | 23 | 59 | 24 | 62 | 24 |
| % of total energy intake | 26a | 5 | 27b | 5 | 27c | 5 | 28d | 4 |
| Saturated fat (g) | 17 | 8 | 19 | 9 | 20 | 9 | 22 | 10 |
| % of total energy intake | 8a | 2 | 9b | 2 | 9c | 2 | 10d | 2 |
| Polyunsaturated fat (g) | 12 | 5 | 13 | 5 | 13 | 5 | 12 | 5 |
| % of total energy intake | 6a | 1·2 | 6a,b | 1·1 | 6b | 1·1 | 6c | 1·1 |
| Monounsaturated fat (g) | 17 | 8 | 19 | 8 | 20 | 9 | 21 | 9 |
| % of total energy intake | 8a | 2 | 9b | 2 | 9c | 2 | 9d | 2 |
| Trans fat (g) | 1·9 | 1·6 | 2·4 | 1·8 | 2·5 | 1·9 | 2·5 | 1·7 |
| % of total energy intake | 0·92a | 0·68 | 1·1b | 0·69 | 1·1b | 0·72 | 1·1b | 0·65 |
| Cholesterol (mg) | 250a,b | 135 | 255a,b | 131 | 250a | 124 | 257b | 116 |
| Linoleic acid (n-6) (g) | 10·4a | 4·2 | 10·9b | 4·2 | 10·9b | 4·4 | 10·7b | 4·4 |
| Linolenic acid (n-3) (g) | 1·3a | 0·5 | 1·4b | 0·5 | 1·4b | 0·6 | 1·4b | 0·6 |
| n-6:n-3 ratio | 7·9a | 1·0 | 7·7b | 1·1 | 7·6c | 0·98 | 7·4d | 1·0 |
| Alcohol (g) | 0·4 | 4·0 | 1·1 | 9·1 | 1·3 | 8·4 | 2·7 | 12·6 |
| % of total energy intake | 0·1a | 1·2 | 0·3b | 2·1 | 0·4b | 2·3 | 0·7c | 3·2 |
a,b,c,dMean values within a row with unlike superscript letters were significantly different (P < 0·05).
Foods that contributed most to energy and macronutrient intakes
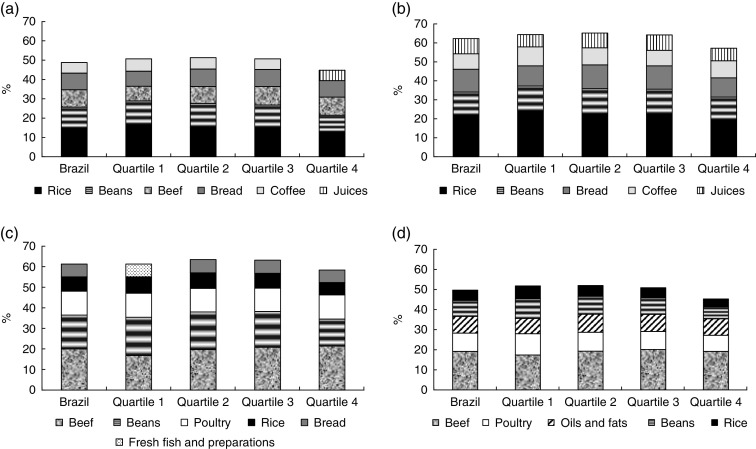
Rice, beans, beef, bread and coffee accounted for >50 % of the daily energy intake in all income quartiles, except for quartile 4, in which ‘juices and refreshments’ replaced coffee among the top five foods that contributed most to energy intake. These foods also contributed substantially to total carbohydrate intake. Rice and beans accounted for 29 % of the daily energy intake among individuals in quartile 1 and for 22 % of the daily energy intake among individuals in quartile 4. Furthermore, rice and beans were among the top five foods with the greatest contributions to the intakes of total carbohydrates, total protein, total fat and fibre (data not shown). Among those in quartile 1, fresh fish represented the food type with the highest contribution to total protein intake (Fig. 1). Beef and poultry represented the foods with the highest contributions to total fat intake (28 %). Coffee, juices/refreshments and soda accounted for an average of 58 % of the added sugar contribution, ranging from 52 % among those in quartile 4 to 64 % among those in quartile 1 (data not shown).
Fig. 1.
Top foods contributing to the intakes of (a) energy, (b) total carbohydrates, (c) total protein and (d) total fat, overall and according to quartile of per capita monthly household income; National Dietary Survey, Brazil, 2008–2009
Discussion
The present study provides a detailed characterization of the energy and macronutrient intakes of Brazilian adolescents, adults and elders according to data collected during the first National Dietary Survey, which was developed in 2008–2009. In general, animal protein, added sugar and saturated fat contributed substantially to total energy intake and trans-fat intake was slightly elevated. There were also reduced intakes of fibre and favourable fats and imbalance in the n-6:n-3 ratio. The analysis revealed significant differences between lowest and highest income quartiles, with the latter reporting higher energy intake levels and striking features of low diet quality, including greater intakes of added sugar and saturated fat. The intakes of rice, beans, beef, bread and coffee accounted for an average of 50 % of the daily energy intake.
The energy intake of adolescents was higher than that of adults and the elderly, particularly among women. This finding might be partially explained by the higher energy demand associated with the rapid growth that is typical during adolescence as well as higher physical activity level( 19 ). The first investigation of dietary habits in the Belgian population, which was conducted in 2004, evaluated 3245 individuals aged ≥15 years using two non-consecutive 24 h recalls combined with an FFQ to obtain data about food consumption( 20 ). Similarly to the present study, the Belgian survey showed that energy intake decreased with age and that the energy intake level of individuals <18 years of age was approximately 35 % higher than that of individuals >75 years of age. Likewise, the first study designed to evaluate energy and macronutrient intakes of schoolchildren from eleven areas in the UK also reported higher energy intakes among adolescents( 21 ).
Energy density can be considered a marker of dietary quality and can be strongly affected by the water and fat content of foods. High-density foods tend to be rich in fats and added sugars and poor in water and fibre( 22 ). Male and female adolescents and those in the two lower income quartiles reported the highest dietary energy densities. It is worth noting that energy density plays an important role in the regulation of food and energy consumption and, in the long term, on individuals’ body weight( 23 ).
Risk factors might be associated with the observed carbohydrate and total fat intake patterns. The consumption of added sugar was high, particularly among adolescents, and sugary drinks such as sodas and juices/refreshments were the greatest contributors to the high levels of added sugar intake. Sugary drinks are known to provide a great deal of energy and large amounts of readily absorbed sugars( 24 ). In contrast, the contributions of total carbohydrate to energy intake reported for studies performed in Belgium (45·8 %), France (44·0–45·5 %) and Hungary (45 % for men and 48 % for women) were smaller than those observed among the Brazilian population( 20 , 25 , 26 ). Nevertheless, in Brazil, total fat intake was within the WHO guidelines (a maximum of 30 % of total energy intake)( 7 ).
However, the lipid profile was unfavourable because the contribution of saturated fat to total energy intake was higher than that recommended by the guidelines, also high trans-fat intake and imbalances in the n-6:n-3 fatty acid ratio were observed. This unfavourable lipid profile was also observed in the Epidemiological Study of Adolescents and Young Adults (ESAY), a study that analysed the food consumption patterns of adolescents and young adults in New Delhi, India( 27 ).
A European study intended to provide a broad view of the health and nutritional status of the European Union (European Health and Nutrition Survey) showed that the energy contribution of total fat exceeded the level recommended by the WHO in all countries included in the study, particularly France, Greece, Portugal, Spain and the UK, and that the intake of polyunsaturated fat was below the recommended levels (6–11 % of energy)( 28 ). The results of the Individual and National Food Consumption Surveys (2006–2007), a French nationwide study that included 1922 individuals ranging from 18 to 79 years of age in which food consumption was recorded through seven dietary records, also reported high levels of energy contribution by total fat, particularly in women (more than 39 %)( 25 ). In England, data from the National Diet and Nutrition Survey (NDNS) of 2008–2009 (n896) revealed high intake of SFA (13–15 % of total energy) and low intake of MUFA (12–13 % of total energy) as well as low intake of trans-fatty acids (0·8 % of total energy)( 29 ). In Finland, polyunsaturated fat intake was about 13 % of total energy for men and 12 % for women( 30 ).
Low polyunsaturated fat intake and high saturated fat intake are associated with various coronary events and an increased risk of death resulting from their effects on plasma lipoproteins( 31 ). According to Mensink et al. ( 32 ), saturated fat contributes to increased LDL-cholesterol levels, whereas polyunsaturated fat leads to decreases in LDL-cholesterol levels. Trans-fats have greater atherogenic effect than do saturated fats; moreover, trans-fats contribute to increased LDL-cholesterol and decreased HDL-cholesterol levels( 33 ). This scenario is worsened by an imbalance in the n-6:n-3 ratio, which was also observed in the present study. Probably, the very low intake of fish contributes to the unfavourable lipid profile in the Brazilian diet( 8 ).
The low fibre intake can be partially explained by the low consumption of wholegrain cereals and fresh fruits and vegetables in Brazil( 8 ). The VIGITEL survey (Surveillance of Risk and Protective Factors for Chronic Diseases Telephone Survey), which was performed in the twenty-six Brazilian states plus the Federal District and included 54 114 adults (≥18 years), revealed low levels of fruit and vegetable consumption, with only 20 % of the population consuming at least five portions of fruits and vegetables daily( 34 ). This low level of fruit consumption can be considered inadequate in terms of health promotion and chronic diseases prevention( 35 ). The greater intakes of fibre, cholesterol, linoleic and linolenic acids among males when compared with females can be explained by the higher energy consumption observed for males.
High intake of protein from animal sources was observed, especially in elderly individuals. Prospective studies conducted in the USA and Europe have shown that high levels of red meat consumption are associated with increased risks of death due to CVD( 36 ) and colorectal cancer( 37 ) and with high levels of oxidative stress biomarkers( 38 ). In Brazil, a population-based study conducted in São Paulo reported that 81 % of men and 58 % of women consumed higher-than-recommended amounts of red meat and excessive red meat consumption among men was associated with a low-quality diet( 39 ).
Given that alcohol consumption was not equally distributed and that the findings reflected population means, energy from alcoholic beverages did not affect the mean total energy intake. Similar observations were found in European countries( 28 ), where alcohol consumption was higher among men than among women. These results were also consistent with those observed in the VIGITEL study, which reported that men consumed excessive amounts of alcoholic beverages (defined as ≥5 drinks/d for men and ≥4 drinks/d for women), nearly threefold higher than women (26 % v. 9 %, respectively)( 34 ).
In general, mean energy intake estimates obtained in the Nationwide Dietary Survey were higher than those observed in the HES. These differences can be explained by the away-from-home food consumption and because of differences in the data collection method. Other important differences were observed: the dietary survey estimates for the contribution of total protein (17 % v. 12·1 %), animal protein (10 % v. 6·7 %) and saturated fat (9 % v. 8·3 %) to total energy intake were greater than those observed in the HES. However, the reverse was observed for the contribution of total carbohydrates (56 % v. 59·2 %), added sugar (13 % v. 16·4 %), total fat (27 % v. 28·7 %) and polyunsaturated fat (6 % v. 9·2 %) to total energy intake.
Rice and beans, which are the major staple foods in Brazil, were important contributors to total energy intake and together comprised 26 % of total dietary energy. Moreover, rice and beans were among the top five contributors to the intakes of total carbohydrates, total fibre, total protein, saturated fat, polyunsaturated fat, and linoleic and linolenic acids.
The study is not free of limitations. Since the consumption of table sugar was indirectly estimated, there is no guarantee that the estimates on sugar intake are unbiased. Furthermore, some degree of under-reporting may be present in the analysed data. The methods used in the survey were validated in a study that used the doubly labelled water method as the gold standard for estimating energy expenditure and the results indicated that under-reporting of energy intake was, on average, 17 %( 8 ). Therefore, it is possible that the degree of inadequacy in the intake of saturated fat and added sugar can be even higher than that estimated in the present study.
On the other hand, several strengths can be recognized in the current study. First, the use of population-based data obtained in a study designed to estimate dietary consumption along the period of an entire year, capturing seasonal variations in Brazilian eating habits( 8 ), is noteworthy. Second, the estimates of usual energy and macronutrient intakes were based on statistical methods performed to appropriately adjust for intra-individual variability; such procedure allowed removal of extreme unlikely values( 8 , 15 ).
Findings from the present study can, at least partly, explain the role of dietary factors in the increased prevalence of overweight and the advance of chronic non-communicable diseases in Brazil. The dietary profile observed in the study can support initiatives aimed at improving the diet quality and reducing the incidence of metabolic disorders and CVD.
Acknowledgements
Financial support: R.A.G.S. received a post-doc scholarship from the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES; grant number 23038.008544/2010-78). R.S. and R.A.P. received research productivity scholarships from the Brazilian National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq). Conflict of interest: None. Authorship: R.A.G.S. participated in the data analysis and manuscript conception, writing and final revision. E.M.Y. participated in the study design and conception, and manuscript conception, writing and final revision. R.S. participated in the study design and conception, and manuscript critical revision. R.A.P. participated in the study design and conception, data analysis, and manuscript conception, writing and final revision. All authors have seen and approved the submitted version of the manuscript and provided approval for the publication of this paper. Ethics of human subject participation: The research protocol was approved by the Ethics Research Committee from the Institute of Social Medicine, University of the State of Rio de Janeiro.
Footnotes
Mate is an infusion prepared from leaves of yerba mate (Ilex paraguariensis) traditionally drunk in the Brazilian South and some states in Central-Western Brazil.
References
- 1. Tessier S & Gerber M (2005) Factors determining the nutrition transition in two Mediterranean islands: Sardinia and Malta. Public Health Nutr 8, 1286–1292. [DOI] [PubMed] [Google Scholar]
- 2. Villareal DT, Apovian CM, Kushner RF et al. (2005) Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr 82, 923–934. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt MI, Duncan BB, Azevedo e Silva G et al. (2011) Chronic non-communicable diseases in Brazil: burden and current challenges. Lancet 377, 1949–1961. [DOI] [PubMed] [Google Scholar]
- 4. Instituto Brasileiro de Geografia e Estatística (2010) Pesquisa de Orçamentos Familiares 2008–2009: antropometria e estado nutricional de crianças, adolescentes e adultos no Brasil. Rio de Janeiro: IBGE. [Google Scholar]
- 5. Instituto Brasileiro de Geografia e Estatística (2004) Pesquisa de Orçamentos Familiares 2002–2003: análise da disponibilidade domiciliar de alimentos e do estado nutricional no Brasil. Rio de Janeiro: IBGE. [Google Scholar]
- 6. Instituto Brasileiro de Geografia e Estatística (2010) Pesquisa de Orçamentos Familiares 2008–2009: avaliação nutricional da disponibilidade domiciliar de alimentos no Brasil. Rio de Janeiro: IBGE. [Google Scholar]
- 7. World Health Organization (2003) Diet, Nutrition and the Prevention of Chronic Diseases. Joint WHO/FAO Expert Consultation WHO Technical Report Series no. 916. Geneva: WHO. [PubMed] [Google Scholar]
- 8. Instituto Brasileiro de Geografia e Estatística (2011) Pesquisa de Orçamentos Familiares 2008–2009: análise do consumo alimentar pessoal no Brasil. Rio de Janeiro: IBGE. [Google Scholar]
- 9. Instituto Brasileiro de Geografia e Estatística (2010) Pesquisa de Orçamentos Familiares 2008–2009: despesas, rendimentos e condições de vida. Rio de Janeiro: IBGE. [Google Scholar]
- 10. Instituto Brasileiro de Geografia e Estatística (2011) Pesquisa de Orçamentos Familiares 2008–2009: tabela de medidas referidas para os alimentos consumidos no Brasil. Rio de Janeiro: IBGE. [Google Scholar]
- 11. Núcleo de Estudos e Pesquisas em Alimentação/Universidade Estadual de Campinas (2011) Tabela Brasileira de Composição de Alimentos (TACO), 4th ed. [NEPA-UNICAMP, editor]. Campinas: BookEditora. [Google Scholar]
- 12. Nutrition Coordination Center (2008) Nutrition Data System for Research (NDSR). Minneapolis, MN: University of Minnesota. [Google Scholar]
- 13. World Cancer Research Fund/American Institute for Cancer Research (2007). Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR. [Google Scholar]
- 14. Instituto Brasileiro de Geografia e Estatística (2011) Pesquisa de Orçamentos Familiares 2008–2009: tabelas de composição nutricional dos alimentos consumidos no Brasil. Rio de Janeiro: IBGE. [Google Scholar]
- 15. Haubrock J, Nöthlings U, Volatier JL et al. (2011) European food consumption validation consortium. Estimating usual food intake distributions by using the multiple source method in the EPIC-Potsdam Calibration Study. J Nutr 141, 914–920. [DOI] [PubMed] [Google Scholar]
- 16. Martin CA, Almeida VV, Ruiz MR et al. (2006) Omega-3 and omega-6 polyunsaturated fatty acids: importance and occurrence in foods. Rev Nutr 19, 761–770. [Google Scholar]
- 17. World Health Organization (2007) Prevention of Cardiovascular Disease: Pocket Guidelines for Assessment and Management of Cardiovascular Risk. Geneva: WHO. [Google Scholar]
- 18. Santos RD, Gagliardi ACM, Xavier HT et al. (2013) I Diretriz sobre o consumo de Gorduras e Saúde Cardiovascular. Arq Bras Cardiol 100, Suppl. 1, S1–S40. [PubMed] [Google Scholar]
- 19. Azevedo MR, Araújo CL, Cozzensa da Silva M et al. (2007) Tracking of physical activity from adolescence to adulthood: a population-based study. Rev Saude Publica 41, 69–75. [DOI] [PubMed] [Google Scholar]
- 20. Temme E, Huybrechts I, Vandevijvere S et al. (2010) Energy and macronutrient intakes in Belgium: results from the first National Food Consumption Survey. Br J Nutr 103, 1823–1829. [DOI] [PubMed] [Google Scholar]
- 21. Gharib N & Rasheed P (2011) Energy and macronutrient intake and dietary pattern among school children in Bahrain: a cross-sectional study. Nutr J 10, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jebbs SA (2005) Dietary strategies for the prevention of obesity. Proc Nutr Soc 64, 217–227. [DOI] [PubMed] [Google Scholar]
- 23. Drewnowski A (2003) The role of energy density. Lipids 38, 109–115. [DOI] [PubMed] [Google Scholar]
- 24. Malik VS, Schulze MB & Hu FB (2006) Intake of sugar sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 84, 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dubuisson C, Lioret S, Touvier M et al. (2010) Trends in food and nutritional intakes of French adults from 1999 to 2007: results from the INCA surveys. Br J Nutr 103, 1035–1048. [DOI] [PubMed] [Google Scholar]
- 26. Sarkadi Nagy E, Bakacs M, Illés E et al. (2012) Hungarian Diet and Nutritional Status Survey – the OTAP2009 study. II. Energy and macronutrient intake of the Hungarian population. Orv Hetil 153, 1057–1067. [DOI] [PubMed] [Google Scholar]
- 27. Gupta N, Shah P, Goel K et al. (2010) Imbalanced dietary profile, anthropometry and lipids in urban Asian Indian adolescents and young adults. J Am Coll Nutr 29, 81–91. [DOI] [PubMed] [Google Scholar]
- 28. Elmadfa I, Meyer A, Nowak V et al. (2009) European Nutrition and Health Report 2009. Forum Nutr 62, 1–405. [DOI] [PubMed] [Google Scholar]
- 29. Pot GK, Prynne CJ, Roberts C et al. (2012) National Diet and Nutrition Survey: fat and fatty acid intakes from the first year of the rolling programme and comparison with previous surveys. Br J Nutr 107, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pietinen P, Paturi M, Reinivuo H et al. (2010) FINDIET 2007 Survey: energy and nutrient intakes. Public Health Nutr 13, 920–924. [DOI] [PubMed] [Google Scholar]
- 31. Salter AM (2013) Dietary fatty acids and cardiovascular disease. Animal 7, Suppl. 1, S163–S171. [DOI] [PubMed] [Google Scholar]
- 32. Mensink RP, Zock PL, Kester ADM et al. (2003) Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 77, 1146–1155. [DOI] [PubMed] [Google Scholar]
- 33. Mensink RP & Katan MB (1990) Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N Engl J Med 323, 439–445. [DOI] [PubMed] [Google Scholar]
- 34. Ministério da Saúde (2012) Vigitel Brasil 2011: Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico. Brasília: MS. [Google Scholar]
- 35. Kumar V, Sinha AK, Makkar HP et al. (2012) Dietary roles of non-starch polysaccharides in human nutrition: a review. Crit Rev Food Sci Nutr 52, 899–935. [DOI] [PubMed] [Google Scholar]
- 36. Pan A, Sun Q, Bernstein AM et al. (2012) Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 172, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Norat T, Bingham S, Ferrari P et al. (2005) Meat, fish, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. J Natl Cancer Inst 97, 906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Montonen J, Boeing H, Fritsche A et al. (2013) Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur J Nutr 52, 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carvalho AM, César CL, Fisberg RM et al. (2013) Excessive meat consumption in Brazil: diet quality and environmental impacts. Public Health Nutr 16, 1893–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]