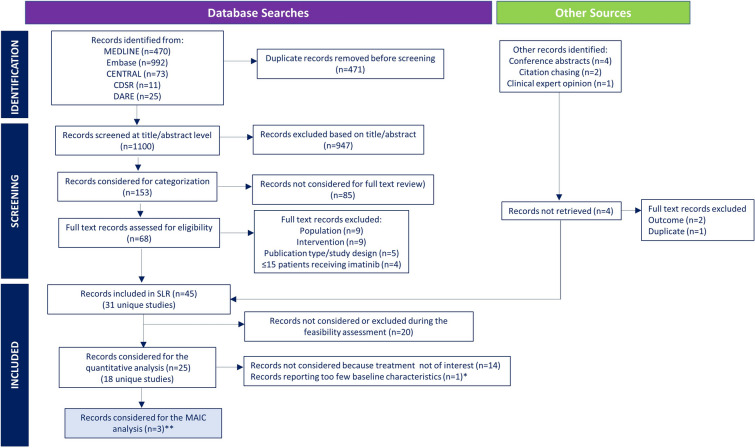
Fig. 1.
Selection of imatinib studies for the MAIC analysis. CDSR Cochrane Database of Systematic Reviews, CENTRAL Cochrane Central Register of Controlled Trials, DARE Database of Abstracts of Reviews of Effectiveness, MAIC matching adjusted indirect comparison, SLR systematic literature review. *Study only reported median age of patients. **The SLR identified 18 unique studies investigating imatinib-based regimens for patients with newly diagnosed Philadelphia chromosome-positive acute lymphocytic leukemia. Studies that did not follow a treatment of interest (i.e., induction without imatinib; not from a German multicenter study group for adult acute lymphoblastic leukemia protocol for hyper-CVAD; imatinib not given continuously) or that reported too few baseline characteristics to allow a meaningful population-adjustment were excluded from the MAIC analysis