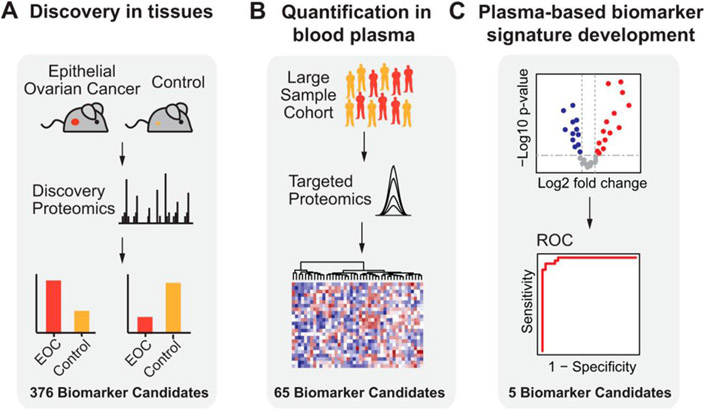
Figure 9:
Overview of study used with permission from Huttenhain et al (2019) with (A) discovery phase in which EOC biomarker candidates were found using a proteomics-based study with EOC tissue sample. (B) Biomarker candidates, which are the plasma-detectable, orthologous human proteins detected to be differentially abundant in (A) were then quantified in plasma samples from a large group of EOC patients along with healthy controls via SRM. (C) The biomarker candidates for EOC detection that were most predictive were chosen, combined in a protein biomarker signature, and submitted to further evaluation in an independent validation set (reprinted with permission from Hüttenhain et al., 2019, copyright 2019 Elsevier).