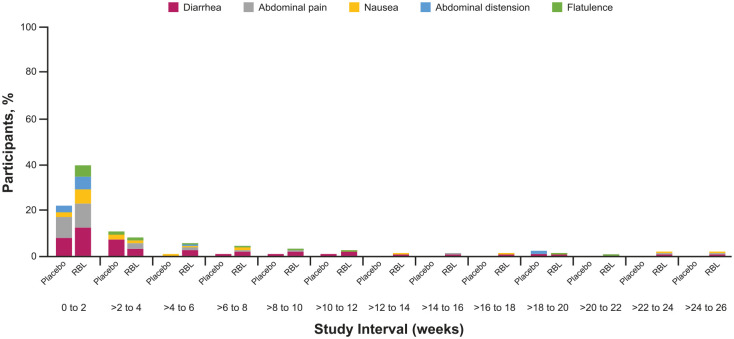
Figure 4.
Gastrointestinal disorder system organ class TEAEs in ⩾5% of participants by onset interval. Percentage of Placebo Only and RBL Only participants who reported gastrointestinal adverse events through 26 weeks after blinded treatment administration. TEAEs were assigned to an onset interval according to their start date; the end date was not considered. A participant with multiple TEAEs coded to the same preferred term (e.g. diarrhea) were counted only once for a given interval, but could occur in multiple time intervals, depending on the onset dates of repeat TEAEs for a given participant.
RBL, fecal microbiota, live-jslm; TEAE, treatment-emergent adverse event.