Figure 2.
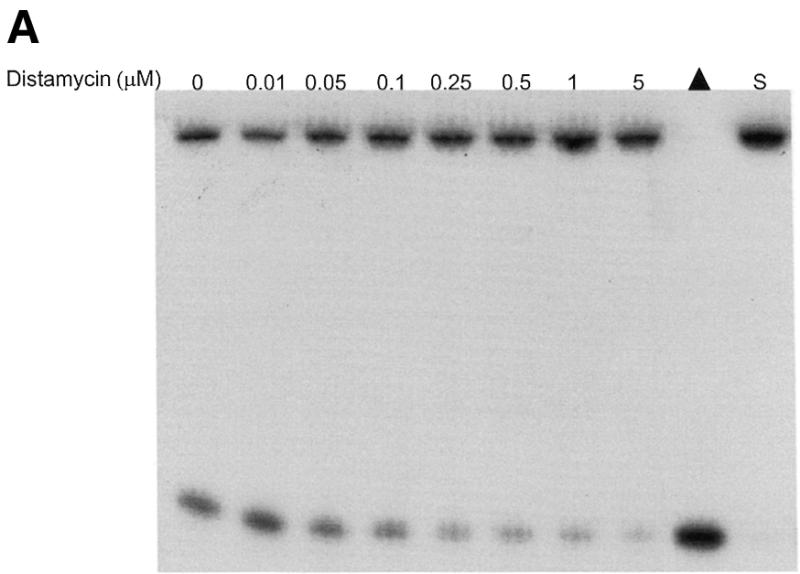
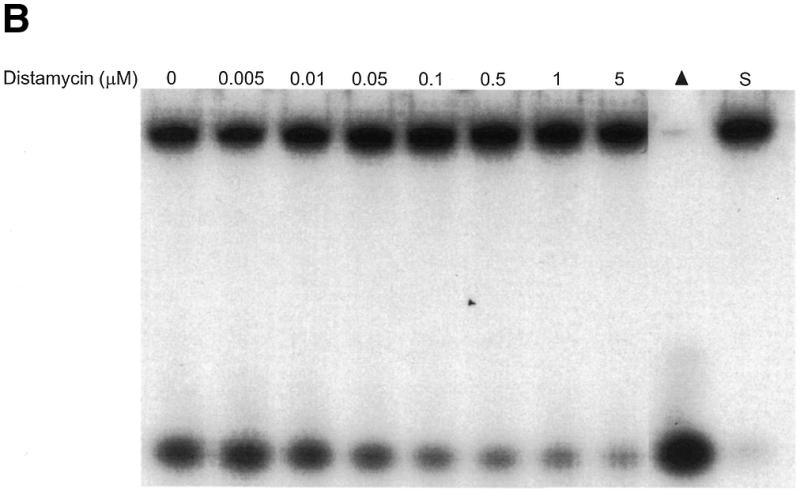
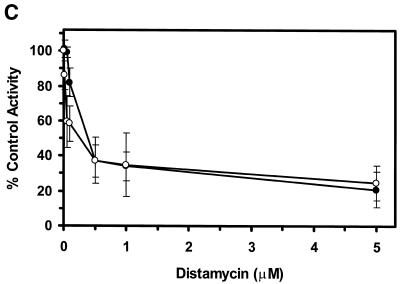
Potent inhibition of WRN and BLM helicase activities on an M13 partial duplex DNA substrate by the minor groove binder distamycin A. WRN protein (96 nM) (A) or BLM protein (19 nM) (B) was incubated with the M13mp18: A-T[5] partial duplex DNA substrate in the presence of the indicated concentrations of distamycin A under the standard helicase reaction conditions as described in Materials and Methods. Incubation was at 24°C for 30 min. Reaction products were analyzed by non-denaturing gel electrophoresis. Representative autoradiographs of WRN and BLM helicase assays are shown. Closed triangle, heat-denatured control; S, no enzyme control. (C) Quantitation of helicase activity (% control activity) as a function of distamycin A concentration. Closed circles, WRN; open circles, BLM. In control reactions, WRN or BLM helicase catalyzed unwinding of ∼50% of the partial duplex substrate. All data points are the average of at least three independent determinations.