FIGURE 1.
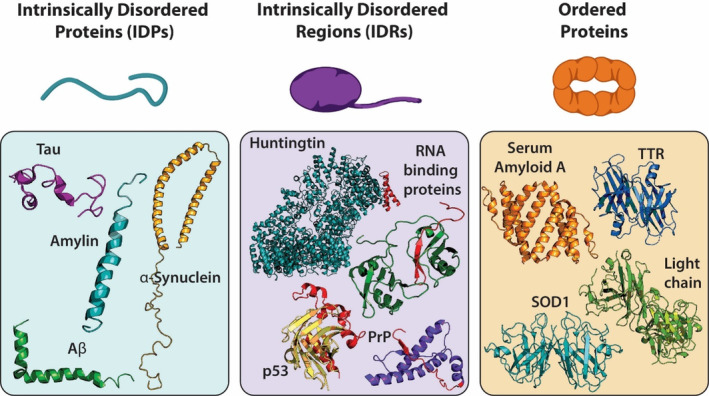
Different types of aggregating proteins. Intrinsically disordered proteins have unstable tertiary or secondary structures such as Tau (PDB 2MZ7), Amylin (PDB 2KB8), Aβ (PDB 1IYT), and α‐synuclein (PDB 1XQ8). Proteins with intrinsically disordered regions have an unstable region that is prone to aggregation, additive mutations typically occur in this region (red region of proteins). Some examples of IDRs are Huntingtin (PDB 6RMH), p53 (PDB 1T4W), PrP (PDB 1QLX), and RNA binding proteins such as FUS and TDP‐43 (PDB 4BS2). Ordered proteins have stable secondary structures which must be destabilized for aggregation to occur such as hexameric Serum Amyloid A (PDB 4IP9, depicts a dimeric piece of the whole structure), tetrameric TTR (PDB 3TFB), dimeric SOD1 (PDB 1SPD), and dimeric antibody light chain (PDB 1YUH).
