|
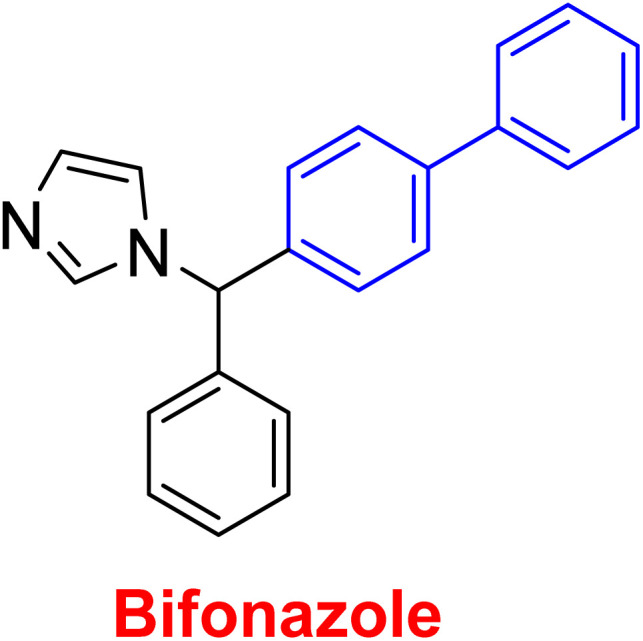
Antibacterial: (Staphylococcus aureus and Salmonella spp.) |
The inducing of chlorine atoms on the biphenyl ring connected to the imidazole ring led to alterations in the physico-chemical properties, therefore these factors can affect the affinity of molecules for the iron of the heme binding site.184 Addition of biphenyl moiety improves its antifungal selectivity, potency and bioisosteric effects185
|
| Therapy for dermatomycoses |
| Antifungal: (Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus)184,185
|
|
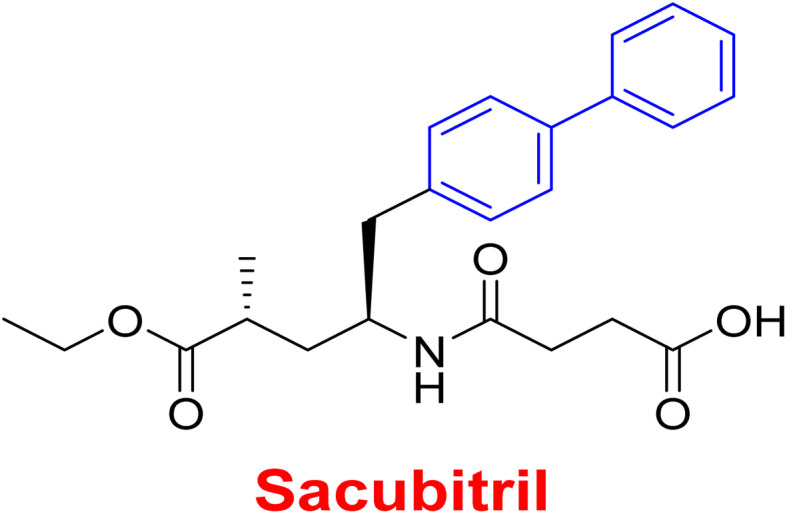
Anti-tubercular: (dormant tuberculosis) |
Due to the presence of substituted biphenyl increases the polarity of the heterocyclic skeleton which might be the reason for high anti-tubercular (anti-TB) activities via interacting with the MurB inhibitors186
|
| Treatment of heart failure with a combination of valsartan |
| Neprilysin inhibitor186,187
|
|
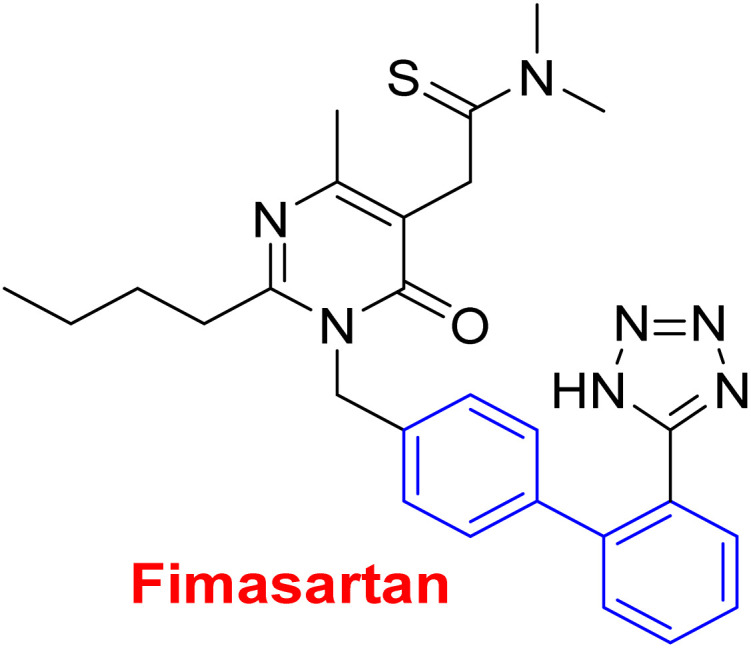
Antihypertensive |
Since inhibition of angiotensin II type 1 (AT1) receptor reduces chronic inflammation associated with hypertension, we evaluated the anti-inflammatory potential and the underlying mechanism of fimasartan188
|
| Anti-inflammatory188
|
|
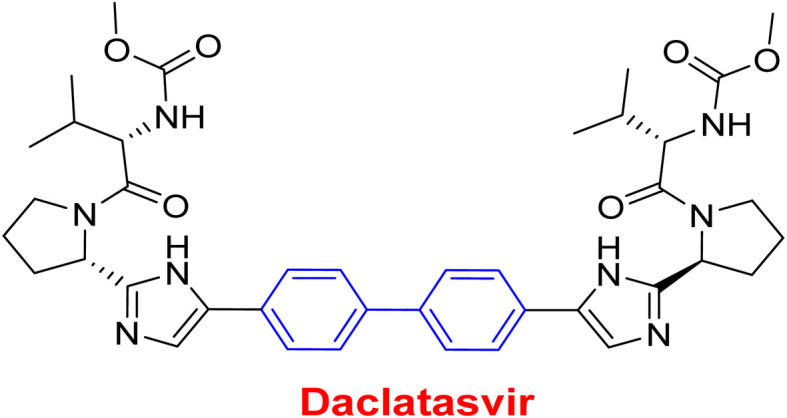
Antiviral |
As a result of sp2 hybridization of CNTs between the drug and target protein sequence, leads to improving the fluorescence reactivity. Because the conjugated system of biphenyl and the presence of Cs/CNT can increase the electroactive surface area of the electrode, leading to an increase in the number of structural flaws189
|
| Treatment selections for hepatitis C virus189,190
|
|
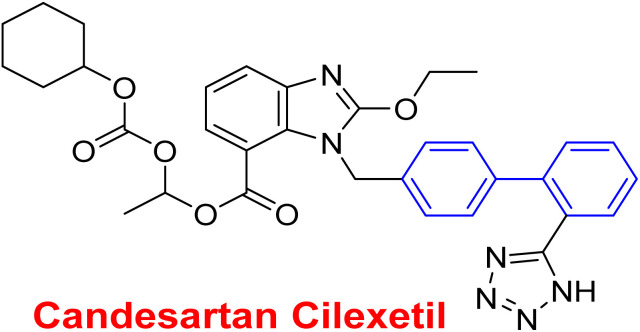
Antihypertensive |
Biphenyl acts as lipophilic moiety in candesartan drug; whereas the candesartan is a selective nonpeptide angiotensin II type 1 (AT1) receptor antagonist which reduces blood pressure effectively.191 Sartans incorporates with membrane receptor in lipid bilayers causing highly transportation possibility of sartans via the receptor192
|
| Active for the AT1 receptor191–193
|
|
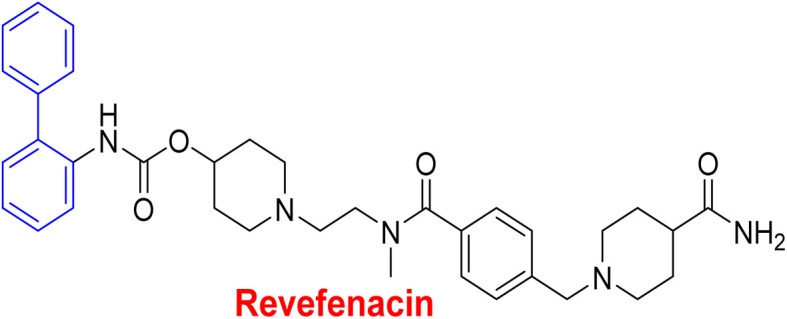
Lung-selective muscarinic cholinergic receptor (mAChR) antagonist |
Biphenyl moiety enhances long-lasting and potency of mediated antagonism of mAChR-causing contraction of human bronchial tissues194
|
| Nebulized inhalation solution to produce long-acting bronchodilation194
|
|
Microsomal triglyceride transfer protein inhibitor |
Biphenyl scaffold for nesting |
| Treatment for human dyslipidemias195
|
|
Anti-human neutrophil collagenase (MMP-8)196
|
Biphenyl residues hit the active site close the catalytic zinc ion that would consequently inhibit the collagenase activity196
|
|
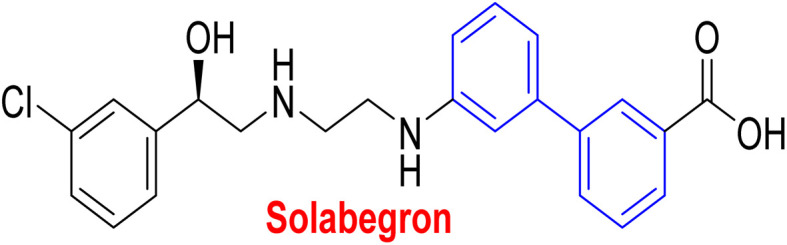
β3-Adrenergic receptor agonist |
RHS of biphenyl ring affords potent human β3-AR agonists with a chlorophenyl ring on the LHS side197
|
| Evokes bladder relaxation |
| Overactive urinary bladder |
| Increases micturition reflex threshold in the dogs197–199
|
|
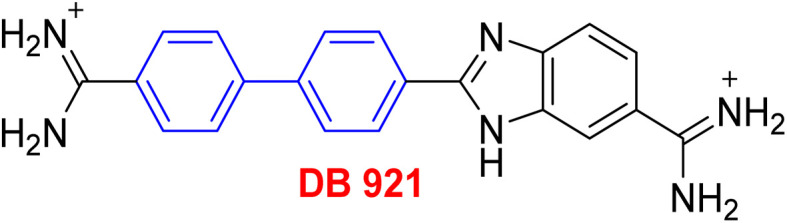
Analysis of water mediated binding in the context of a DNA complex |
The interactions of the molecules containing of biphenyl with DNA AT sites increasing DNase I footprinting depending on increasing conjugation process which enhancing biosensor-surface plasmon resonance, circular dichroism microcalorimetry, and isothermal titration200
|
| Promising agent against parasites |
| Change in AT sequences with destruction of the kinetoplast and cell death200
|
|
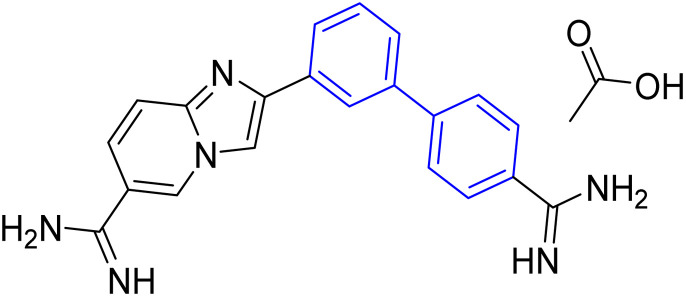
Antiprotozoal |
Biphenyl scaffold for nesting |
| Anti-trypanosomal201
|
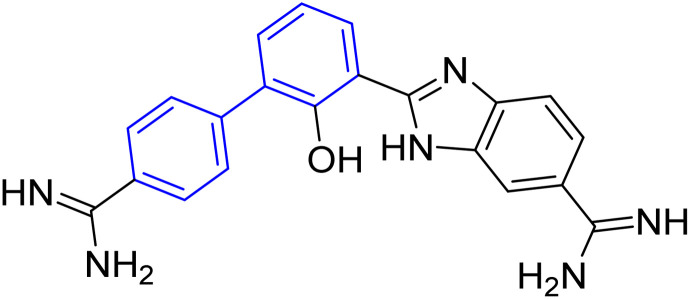
|
Anti-protozoan202
|
Biphenyl scaffold for nesting |
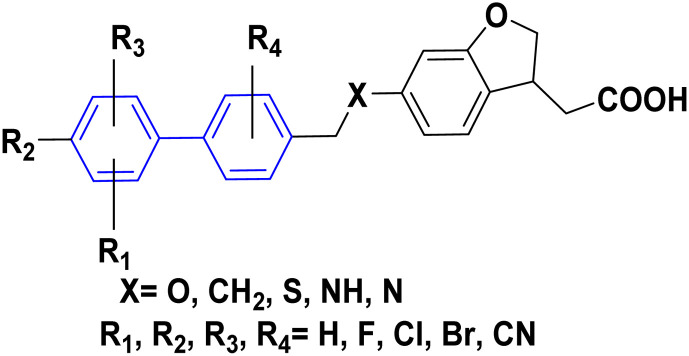
|
Treating diabetes mellitus203
|
Biphenyl scaffold for nesting |
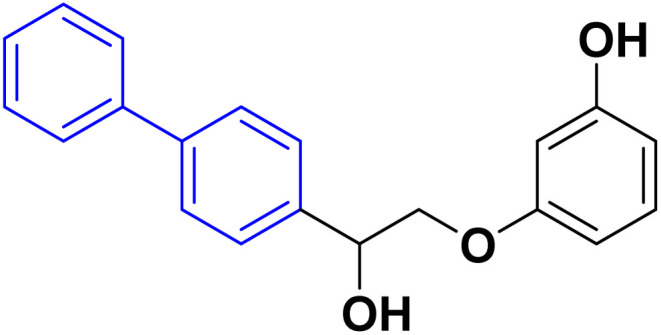
|
Treatment CNs impairments involving major depressive disorder204
|
Biphenyl-based NMDA negative allosteric modulator (NAM) has low affinity for the human ether-a-go-go-related gene ion channel (hERG) and the dynamics calculations suggest a various binding mode (ifenprodil-like) compared to another biaryl-based NMDA NAM EVT-101 204
|














