Figure 4.
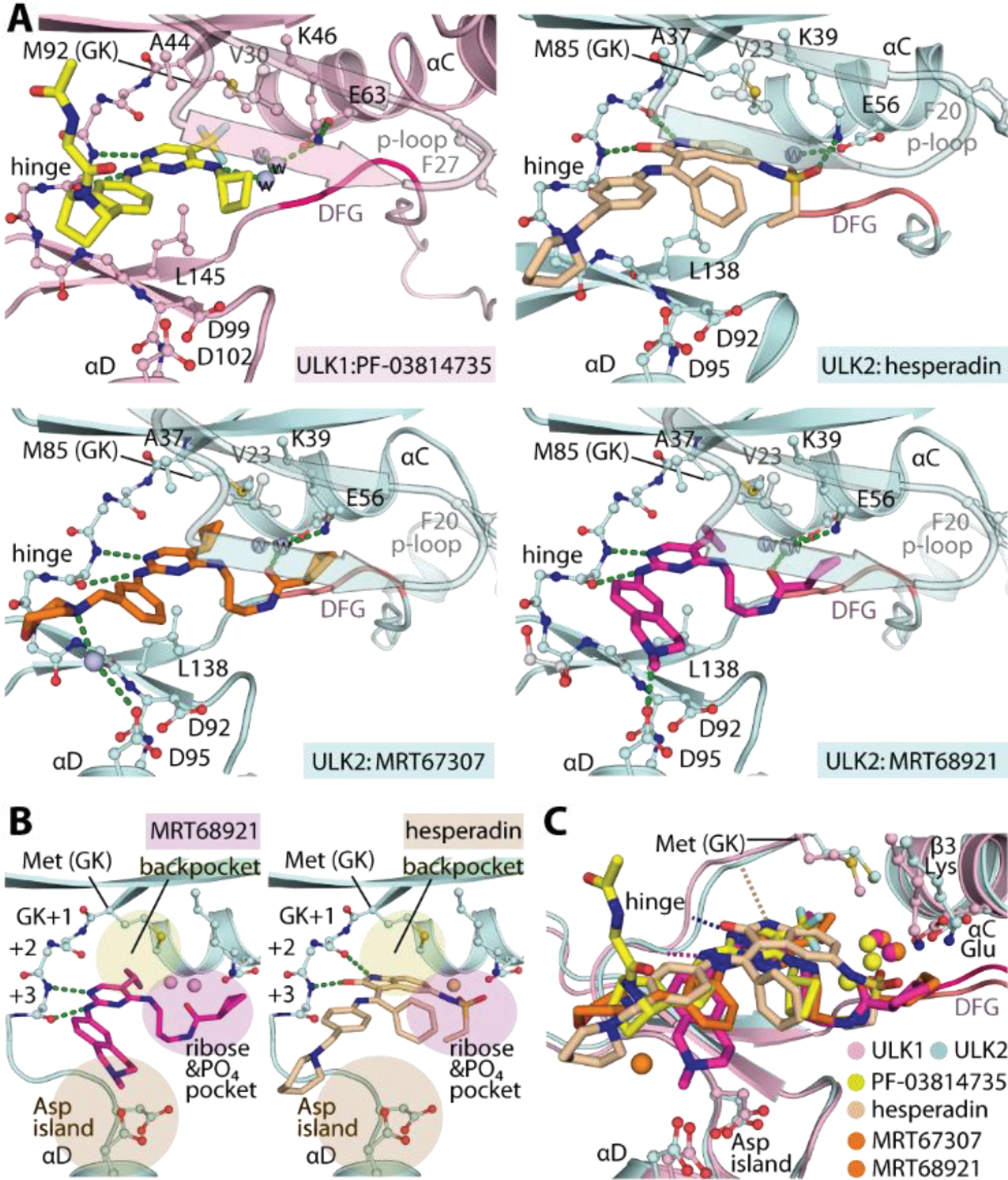
Crystal structures of ULK1/2-inhibitor complexes. A) Detailed interactions between ULK1 and PF-03814735 and ULK2 with hesperadin, MRT67307 and MRT68921. Bound water molecules are shown in spheres. B) Schematic illustrations of the binding pockets of the 2-aminopyrimidine-based MRT68921 and 2-oxindole-based hesperadin revealing different interactions of the hinge binding motifs. Three cavities for accommodation of the inhibitors in ULK1/2 are highlighted. C) Superimposition of ULK1/2-inhibitor complexes demonstrated highly similar binding sites in both ULK kinases and also the commonly occupied space by diverse inhibitors.
