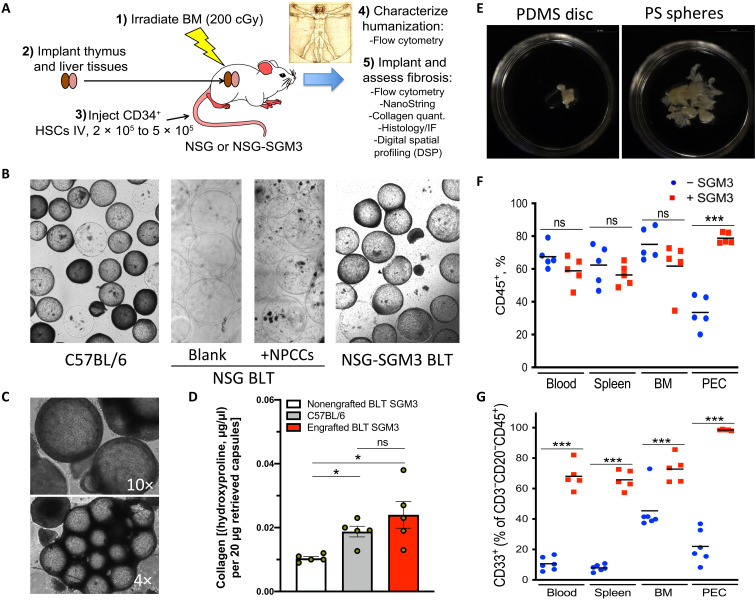
Fig. 1. Identifying a humanized model capable of biomaterial fibrosis.
500-μm-diameter SLG20 alginate spheres (0.5-ml volume) were implanted into the IP space of C57BL/6 and various humanized mouse models for 28 days and then analyzed for the degree of fibrosis. (A) Graphic showing steps required to achieve fibrotic FBR in the human HSC–engrafted NSG-SGM3 BLT model. BM, bone marrow; IV, intravenous. (B) Bright-field images of retrieved spheres reveal significant overgrowth in control C57BL/6 mice (left), with no fibrosis in NSG BLT mice, without or with xenogeneic neonatal pig cell clusters (NPCCs) (middle images). FBR was only observed after transgenic expression of three human cytokines [SCF, GM-CSF, and IL-3 (SGM3)] for innate immune cell maintenance. (C) Bright-field images (4× and 10×) of fibrosed spheres retrieved from engrafted NSG-SGM3 BLT mice 28 days after implantation. (D) Collagen levels on retrieved capsules by hydroxyproline assay from nonengrafted NSG-SGM3 BLT (white), wild-type C57BL/6 (gray), or engrafted (humanized) NSG-SGM3 BLT mice (red). (E) Phase contrast images of fibrosed and epididymal fat-embedded 5-mm PDMS discs and 500-μm PS spheres retrieved 28 days after IP implantations (alginate in fig. S1B). (F) Flow cytometry analysis reflected similar CD45+ engraftment without (blue) versus with (red) SGM3 incorporation in the blood, spleen, and bone marrow; however, a significant increase occurred in the IP space. PEC, peritoneal exudate cell. (G) With SGM3 adoption, CD45+CD3−CD20−CD33+ innate myeloid lineages were significantly increased in all compartments. For statistical analysis, one-way analysis of variance (ANOVA) with Bonferroni multiple comparison correction was used. *P < 0.05 and ***P < 0.0001; ns, not significant. n = 5 (biologic replicates) per group. Experiments were repeated three times.