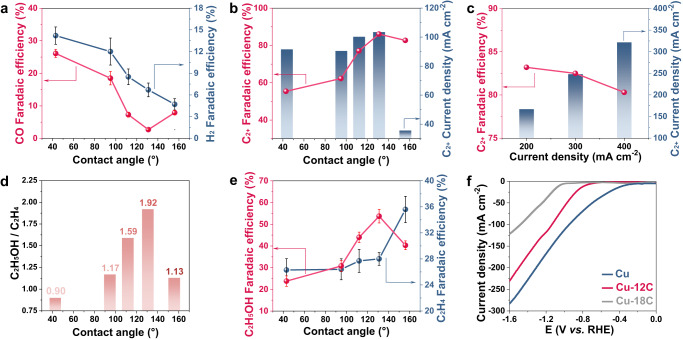
Fig. 2. Effect of controllable wettability on CO2 electroreduction performance.
a Faradaic efficiencies of CO and H2. b Faradaic efficiencies and partial current densities of C2+ on Cu electrodes in 1 M KOH (aq.) at −1.2 V versus (vs.) reversible hydrogen electrode (RHE) with various contact angles c Faradaic efficiencies and partial current densities of C2+ on Cu-12C under different current densities. d Ethanol to ethylene ratios and e Faradaic efficiencies of ethanol and ethylene on Cu electrodes in 1 M KOH (aq.) at −1.2 V vs. RHE with various contact angles (without iR correction). It is demonstrated that the strong dependence of the C2 product distribution on the wettability of Cu catalyst. The evolution of H2 and CO is suppressed via alkanethiol treatment. f Linear sweep voltametric (LSV) curves of the Cu electrodes with and without alkanethiol modification in 1 M KOH (ν = 50 mV s–1), showing the lowered current of the hydrophobic-treated Cu catalyst at a given potential. Error bars represent the standard deviation from at least three independent measurements.