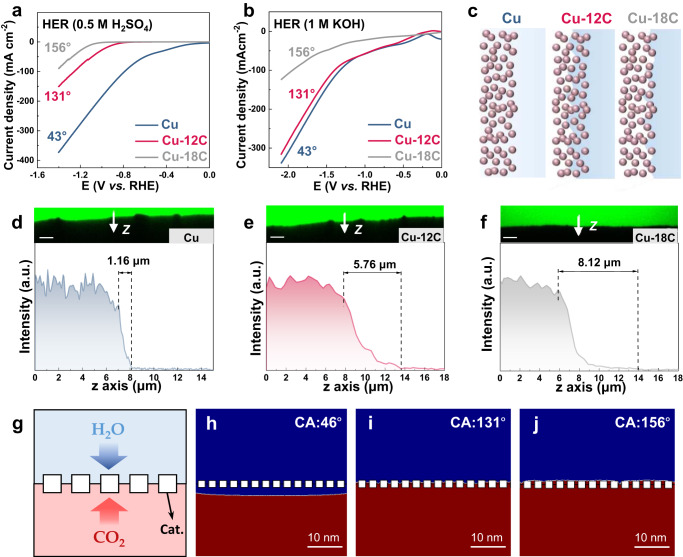
Fig. 4. Effect of controllable wettability on *H coverage via H2O mass transport.
a LSV curves of H2 evolution on the Cu electrodes with different contact angles in 0.5 M H2SO4 electrolyte and b 1 M KOH electrolyte (ν = 50 mV s–1). The hydrophilic Cu electrode (CA: 43°, blue line) exhibits optimal hydrogen evolution activity, suggesting efficient H2O transport in a hydrophilic environment. c Schematic illustration of available H2O concentration at reaction interface of Cu, Cu-12C, and Cu-18C, respectively. The white and blue parts in the schematic represent gas and liquid, respectively. The balls represent catalysts. The catalyst layers in the hydrophilic (Cu) and super-hydrophobic state (Cu-18C) are dominated by the liquid-solid interface and the gas–solid interface, respectively. The catalyst layer with balanced wettability (Cu-12C) is occupied by gas–liquid–solid interface. d–f Cross-sectional fluorescence images (scale bar: 20 μm) and corresponding z-axis fluorescence intensity line scans of labeled regions (white arrows) of Cu, Cu-12C, and Cu-18C, respectively. The decay distance of the fluorescence intensity increases with the improvement of the hydrophobicity of the catalyst layer, indicating the smaller available H2O concentration. g Schematic of the CFD simulation. The red and blue parts in the schematic represent gas and liquid, respectively. The squares represent catalysts. The gaps between the squares represent the pores between the real catalyst islands. The different wettability of the catalyst layer will lead to differences in the interfacial contact state and the local CO2/H2O ratio. Cat. catalyst. h–j Comparison of the modeled gas–liquid mass transport with different interfacial wettability, in which the red color in the simulation result represents a CO2 volume fraction of 100% (electrolyte 0%), while the blue color represents a CO2 volume fraction of 0% (electrolyte 100%). The CO2/H2O ratio at the reaction interface increases with the enhance of hydrophobicity. CA: contact angle. The a.u. stands for arbitrary units.