Summary
The spinal cord is a part of the central nervous system located within the spinal canal of the vertebrae. Here, we present a protocol to prepare mouse spinal cord sections for patch-clamp and histology experiments. We describe steps for isolating spinal cord from the spinal canal and obtaining acute slices for patch-clamp experiments. For histology experiments, we detail fixing spinal cord for cryosectioning and imaging. This protocol provides procedures to assess neuronal activity and protein expression of sympathetic preganglionic neurons.
For complete details on the use and execution of this protocol, please refer to Ju et al.1
Subject areas: Metabolism, Neuroscience
Graphical abstract

Highlights
-
•
Protocol describes how to prepare mouse spinal cords for various analyses
-
•
Spinal cord is isolated and sectioned using vibratome for patch-clamp experiments
-
•
Spinal cord is cryosectioned and stained on a slide glass for histology experiments
-
•
Protocol can be used to assess activity and protein expression of spinal cord neurons
The spinal cord is a part of the central nervous system located within the spinal canal of the vertebrae. Here, we present a protocol to prepare mouse spinal cord sections for patch-clamp and histology experiments. We describe steps for isolating spinal cord from the spinal canal and obtaining acute slices for patch-clamp experiments. For histology experiments, we detail fixing spinal cord for cryosectioning and imaging. This protocol provides procedures to assess neuronal activity and protein expression of sympathetic preganglionic neurons.
Before you begin
This protocol describes how to prepare solutions, materials, and instrument settings to obtain spinal cord slices from mice. The spinal cord slices can be used to study the anatomy and physiology of spinal cord neurons including sympathetic preganglionic neurons (SPNs) within the intermediolateral column (IML). The current protocol is an adaptation of methods described previously.1,2 With some modification, this protocol can also be applied to study α-motor neurons within the anterior horn or sensory neurons within the posterior horn.
Institutional permissions
All procedures were conducted according to the KAIST Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (Protocol No. KA2021-126).
Preparation of artificial cerebrospinal fluid (ACSF) solutions (for patch-clamp experiments)
Timing: 90 min
-
1.
Composition of ACSF is described below in “materials and equipment”.
-
2.
Firstly, make “modified” ACSF for making spinal cord slices. Adjust pH to 7.30–7.40 with carbogen gas (95% O2 and 5% CO2).
-
3.
When the pH is stable, store the modified ACSF at –80°C for about 30 min until the modified ACSF reaches a slush-like consistency.
-
4.
Then, make ACSF for storage and recording. Adjust pH to 7.30–7.40 with carbogen gas.
-
5.
When the pH is stable, pour the oxygenated ACSF into a lattice-shaped storage chamber and recording solution chambers. Place the chambers in a 34°C water bath and supply carbogen gas throughout the experiments.
Preparation of agarose block (for patch-clamp experiments)
Timing: 40 min
-
6.
Using a microwave, prepare 100 mL of 3.0% agarose solution in a glass flask or beaker.
-
7.
Fill up a 100 mm Petri dish (1.5 cm depth) with the agarose solution.
CRITICAL: For safety, always wear heat-resistant gloves when handling heated glass flask or beaker.
-
8.
Wait for about 30 min until the agarose gel hardens completely.
- 9.
Figure 1.
Preparation of agarose block and storage chamber for patch-clamp experiment
(A) Triangular prism-shaped block cut from hardened 3.0% agarose gel in a 100 mm Petri dish. The length of the block is 3.0–3.5 cm, which is enough to accommodate spinal cords.
(B) Cross section of the block. One edge is trimmed concave to apply glue and better hold the spinal cord.
(C) Top view of an 8 × 8 acrylic lattice with mesh attached at the bottom. Inner dimension of a cell, 12 × 12 × 20 mm.
(D) Top view of an acrylic frame with 8 acrylic pods (10 × 10 × 10 mm) attached at the bottom.
(E) Assembled acrylic lattice and frame is placed in an acrylic chamber.
VibroCheck and vibratome setting (for patch-clamp experiments)
Timing: 10 min
-
10.
To ensure an optimum position of the blade and minimize the vertical vibration, perform VibroCheck protocol according to the manufacturer’s instruction.
Preparation of solutions (for histology experiments)
Timing: 30 min
-
11.Preparation of blocking and permeabilization solution.
-
a.Make 0.3% PBST solution with sterile 1x PBS solution and Triton-X.
-
b.Dissolve normal donkey serum to make 5% normal donkey serum in 0.3% PBST. Typically, 3,000 μL of 0.3% PBST solution and 150 μL of normal donkey serum are required for serial immunofluorescence stainings of ChAT and Fos from the spinal cord of a mouse (Table 1).
-
c.We stored this blocking and permeabilization solution at 4°C and used it within two weeks.
-
a.
-
12.Preparation of sodium citrate buffer solution for heat-induced antigen retrieval
-
a.Add 2.94 g trisodium citrate dihydrate to 1 L ddH2O.
-
b.Titrate the pH to 6.0 with 1M HCl. The solution can be stored at 15°C–25°C for 3 months or at 4°C for 6 months.
-
a.
Table 1.
Steps for serial immunofluorescence staining of ChAT and Fos
| Step | Reagent and dilution condition | Required time and storage condition |
|---|---|---|
| Blocking and permeabilization | 5% normal donkey serum in 0.3% PBST | 1 h at 15°C–25°C |
| Primary antibody | Goat anti-ChAT Ab 1:100 | 18–24 h at 4°C |
| 5-min wash, three times | ||
| Secondary antibody | Donkey anti-Goat 488 1:200 | 1.5 h at 15°C–25°C |
| 5-min wash, three times | ||
| Primary antibody | Rabbit anti-Fos Ab 1:500 | 18–24 h at 4°C |
| 5-min wash, three times | ||
| Secondary antibody | Donkey anti-Rabbit 647 1:500 | 1.5 h at 15°C–25°C |
| 5-min wash, three times | ||
| DAPI | 1:10,000 | 10 min at 15°C–25°C |
| 5-min wash, three times | ||
| Coverslip mounting | N.A. | N.A. |
N.A. not applicable.
Then, go to step 1 of “step-by-step method details” section.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-c-Fos antibody | Abcam | ab190289 |
| Goat anti-ChAT antibody | Chemicon | AB144P |
| Donkey anti-goat plus 488 | Invitrogen | A32814 |
| Donkey anti-rabbit plus 647 | Invitrogen | A31573 |
| Chemicals, peptides, and recombinant proteins | ||
| Isoflurane | Piramal Critical Care | Isoflurane |
| Agarose | E&S | HB0100500 |
| Sucrose | Sigma-Aldrich | S9378 |
| Sodium bicarbonate (NaHCO3) | Sigma-Aldrich | S5761 |
| Potassium chloride (KCl) | Fisher Scientific | P/4240/60 |
| Sodium phosphate monobasic (NaH2PO4) | Sigma-Aldrich | S0751 |
| Magnesium chloride (MgCl2) | Sigma-Aldrich | M0250 |
| Calcium chloride (CaCl2) | Sigma-Aldrich | C3881 |
| D-(+)-Glucose | Sigma-Aldrich | G7528 |
| Sodium chloride (NaCl) | Sigma-Aldrich | S7653 |
| Magnesium sulfate (MgSO4) | Sigma-Aldrich | 230391 |
| 4% Paraformaldehyde | FUJIFILM Wako Pure Chemical Corporation | 163-20145 |
| Trisodium citrate dihydrate | Sigma-Aldrich | S1804 |
| Triton-X | Calbiochem | 9440 |
| Normal donkey serum | Jackson ImmunoResearch | 017-000-121 |
| Hydrochloric acid (HCl) | Samchun | H0255 |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J, male, 6–10 weeks old | The Jackson Laboratory | 000664 |
| Mouse: ChAT-IRES-Cre | The Jackson Laboratory | 006410 |
| Mouse: Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J | The Jackson Laboratory | 007914 |
| Other | ||
| Stereomicroscope | Korea Lab Tech | Optinity KS-200 |
| Fine forceps | Dumont | 11295-10 |
| Micro-dissecting scissors | Biomedical Research Instruments | 11-1390 |
| Graefe tissue forceps | Roboz Surgical Instrument | RS-5153 |
| Petri dish | SPL | 10100 and 11060 |
| 30 mL syringe | Korea Vaccine | 21G-30ML |
| 23-gauge sterile needle | Korea Vaccine | 23G-1 |
| Vibratome | Leica | VT1200S |
| Super glue (cyanoacrylate adhesive) | Henkel | Loctite 401 |
| Stainless blade (razor blade) | Dorco | ST-300 |
| Water bath | JS Research | JSWB-11T |
| Cryostat | Leica | CM1950 |
| Embedding medium (O.C.T. compound) | Leica | Surgipath FSC22 |
| Slide glass | Electron Microscopy Sciences | 63700-W1 |
| ImmEdge Pen | Vector Laboratories | H-4000 |
| Peel-A-Way embedding molds | Sigma-Aldrich | E6032 |
| Brush for mounting | Hwahong | Hwahong 948, #1 |
Materials and equipment
Lattice-shaped storage chamber
-
•
8 × 8 acrylic lattice is attached to a mesh bottom (Figure 1C). The lattice is fitted on an acrylic frame with 8 acrylic pods (Figure 1D), and is placed in an acrylic chamber (Figure 1E). For the acrylic lattice, 2 mm-thick acryl (inner frames) and 5 mm-thick acryl (outermost frame) was used. For the acrylic frame and chamber, 5 mm-thick acryl was used.
ACSF2 for storage and recording
| Reagent | Final concentration (mM) | Amount (g/1L ddH2O) |
|---|---|---|
| NaCl | 126 | 3.594 |
| NaHCO3 | 26 | 1.092 |
| KCl | 2.8 | 0.104 |
| NaH2PO4 | 1.25 | 0.075 |
| MgSO4 | 1.2 | 0.148 |
| CaCl2 | 2.5 | 0.184 |
| Glucose | 5 | 0.451 |
Solution was freshly prepared right before the experiments and were not stored or reused.
Modified ACSF2 for slicing
| Reagent | Final concentration (mM) | Amount (g/500 mL ddH2O) |
|---|---|---|
| Sucrose | 220 | 37.0 |
| NaHCO3 | 26 | 1.092 |
| KCl | 2.5 | 0.093 |
| NaH2PO4 | 1 | 0.060 |
| MgCl2 | 5 | 0.508 |
| CaCl2 | 1 | 0.074 |
| Glucose | 10 | 0.901 |
Solution was freshly prepared right before the experiments and were not stored or reused.
Alternatives: The composition of the ACSF solutions can be modified according to the purpose of the experiments.
Step-by-step method details
Spinal cord preparation (for patch-clamp and histology experiments)
Timing: 15–30 min
This step will isolate spinal cords from a fully anesthetized mouse. We used ChAT-IRES-Cre::tdTomato reporter mice to identify the SPNs using a fluorescence microscope attached to a patch-clamp setup or a confocal microscope. For histology experiments, SPNs of wild-type mouse can be labeled with immunofluorescence staining with anti-ChAT antibodies.
-
1.
Anesthetize the mouse with 5% isoflurane until righting reflex and pedal withdrawal reflex are absent.
-
2.
After fixing the four limbs to the surgery table in a supine position, expose the liver and heart with Y-incision from midline abdomen to chest.
-
3.Transcardial perfusion troubleshooting 1
-
a.Make a small incision at the right atrium.
-
b.Use a 30 mL disposable syringe and a 23-gauge beveled syringe needle to perfuse fluid into the left ventricle at the apex (Figure 2A)
-
i.For patch-clamp experiments, perfuse 20 mL of ice-cold modified ACSF solutions.
-
ii.For histology experiments, perfuse 20 mL of normal saline followed by 20 mL of 4% PFA.
-
i.
-
a.
Note: Transcardial perfusion is successful if the color of the liver turns from deep brown to beige.
-
4.Preparation of the mouse spinal cord.
-
a.Lay the mouse in a prone position and fix the four limbs to the surgery table.
-
b.Decapitate to expose a cross section of the cervical spinal cord within the spinal canal. It is recommended to cut the cervical vertebra (and the spinal cord) orthogonally.Note: The spinal canal lies dorsal to the vertebral body, medial to the pedicle and the transverse process, and ventral to the lamina and the spinous process.
-
c.Make an incision in the midline of back skin from the level of decapitation to the rostral end of the tail.
-
i.Pull the skin apart to both sides.
-
ii.Remove the interscapular brown adipose tissue and adjacent muscles covering the thoracic vertebrae.
-
i.
-
d.Gently cut both laminae of the vertebrae with micro-dissecting scissors from cervical to upper lumbar level. Perform laminectomy to upper lumbar level while grabbing resected dorsal part including spinous process and laminae with forceps in the other hand (Figure 2B).
-
e.Make an oblique cut of the lumbar vertebra (and the spinal cord) at the level between the origin of the 2nd and the 3rd ribs from the caudal end. Oblique cutting helps to distinguish lumbar ends from cervical ends (Figures 2C and 2D).Note: IML containing SPNs is found in spinal cord levels from T1 to L1. The L1 level lies within the spinal canal of the T11 vertebrae which is the origin of the 3rd rib from the caudal end.
-
f.To remove the spinal cord out of the spinal canal, gently grab and lift up the cervical end of the spinal cord with forceps. Cut spinal roots at both sides with micro-dissecting scissors and proceed to the L1 level (Figure 2E).
-
g.Place the spinal cord in a 100 mm Petri dish filled with either modified ACSF (ice-cold, for patch-clamp experiments) or 4% PFA (at 15°C–25°C, for histology experiments).
-
a.
-
5.Trimming the spinal cords
-
a.Under a stereomicroscope, peel off the spinal meninges and spinal nerve roots covering the spinal cord using fine forceps (Methods video S1).
-
b.Grab the cervical end with fine forceps to find and peel off meninges and nerve roots. The cervical end will be cut off in the next step.
-
a.
CRITICAL: Spinal meninges are tough membranes that can cause strangulation injury to the spinal cord. To prevent strangulation, tear the meninges at the cervical end and peel off longitudinally as shown in Methods video S1. If the spinal cord is strangulated while peeling spinal meninges off, the meninges at the area of strangulation must be eased off with fine forceps.
Figure 2.
Dissection of mouse spinal cord
(A) A schematic illustration of transcardial perfusion.
(B) Laminectomy is performed by gently cutting laminae at 3 and 9o’clock directions from cervical to lumbar level.
(C) Oblique cutting of the lumbar vertebra (and the spinal cord).
(D) A schematic illustration demonstrating the level of oblique cutting.
(E) Gentle cutting of spinal nerve roots on both sides and removing the spinal cord from spinal canal.
Making acute spinal cord slices with a vibratome (for patch-clamp experiments)
Timing: 60 min
This step prepares acute spinal cord slices for patch-clamp recording. To reliably obtain slices of constant thickness from this fragile tissue, attach the spinal cord to an agarose block.
-
6.
Prior to this step, the lattice-shaped storage chamber should be filled with storage solutions and the VibroCheck should be performed, as described in “before you begin” section.
-
7.
Place trimmed spinal cord submerged in ice-cold modified ACSF (from step 3 of “before you begin” section) in a dry Petri dish. Make sure the spinal cord lies straight and remove excess water from the surface with filter paper.
CRITICAL: Use the tip of paper towel or filter paper so that the papers may not adhere to the spinal cord. Removing excess water from the surface is an important step to ensure that glue attaches the spinal cord tightly to the agarose block.
-
8.
Apply glue on the concave edge of the agarose block (Figure 3A), and gently place the spinal cord on the glued edge of the block (Figure 3B). During this step, grab both ends of the spinal cord with fine forceps. Wait 5 min after placing the spinal cord on the agarose block (Figure 3B).
CRITICAL: Proper amount of glue is another key point. Excessive amount of glue not only requires more time to dry but also produces sticky ends, which makes it difficult to slice with a vibratome. Insufficient amount of glue will lead to detachment of the spinal cord from the agarose block.
-
9.
Cut off the cervical and lumbar ends of the spinal cord using razor blades, leaving ∼1.8 cm spinal cord and agarose block. To preserve the T1 level of spinal cord, the remaining spinal cord must include the narrowing part of the cervical enlargement (Figures 3C–3E). The orientation of spinal cord should be maintained before cutting the spinal cord and agarose block.
Note: Spinal cord has cervical and lumbar enlargements. In the rostral to caudal direction, the narrowing of the spinal cord means the beginning of the thoracic spinal cord, and the widening of spinal cord means the beginning of the lumbar spinal cord.
-
10.
Place the agarose block upright with the lumbar part facing down and glue it to the specimen plate.
-
11.
Wait for 5–10 min for attachment.
-
12.
Prior to sectioning, make V-shaped incision along the longitudinal axis of the agarose block to minimize the moving distance of blade (Figure 3F).
-
13.
Place the specimen plate in buffer tray of the vibratome and pour ice-cold modified ACSF into the buffer tray (Figure 3G).
-
14.
Obtain spinal cord sections following the manufacturer’s instruction; speed 0.10–0.20 mm/s, thickness 250 μm. When the blade reaches the V-shaped incision, use forceps to gently push the agarose from the other side of block against the blade so that the agarose block and spinal cord slices can be easily released. Troubleshooting 2.
-
15.
Transfer sections immediately to storage chambers filled with oxygenated ACSF (Figure 3H).
-
16.
Allow 1 h for recovery in the storage solution at 34°C before starting patch-clamp experiments. Troubleshooting 3.
Note: Based on MRI image of the mouse spinal cord, it is 18.2 mm from T1 to L1 level. The length of the T1 level is half the length of each of T2 to L1 levels.3 Theoretically, 18.2 mm-long spinal cord would contain 72.8 slices when cut in sections with 250 μm thicknesses. Therefore, we assumed that the slices consist of 2.7 sections of T1, and 5.4 sections of each of T2 to L1. The IML region of the ChAT-IRES-Cre::tdTomato reporter mouse was explored in the rostral to caudal direction, and the first slice where the tdTomato-positive neurons were found was regarded as T1 (Figures 4A and 4B).
Figure 3.
Making acute spinal cord slices using a vibratome
(A) Applying glue on the concave edge of the agarose block.
(B) Laying down the spinal cord on the agarose block using fine forceps.
(C) A schematic illustration of cervical and lumbar enlargements of mouse spinal cord.
(D) Oblique view of agarose block with spinal cord. Inset shows a different view of agarose block with spinal cord.
(E) Cutting of the agarose block to remove cervical and lower lumbar ends of the spinal cord. The ∼1.8 cm-long thoracolumbar spinal cord in the center is used for making spinal cord slices.
(F) Agarose block is glued upright on the specimen plate. Black arrow indicates the V-shaped incision line (black line).
(G) The specimen plate and the agarose block with spinal cord is placed in the buffer tray. Black arrow indicates the V-shaped incision line (black line).
(H) Acute spinal cord sections collected in the storage chamber.
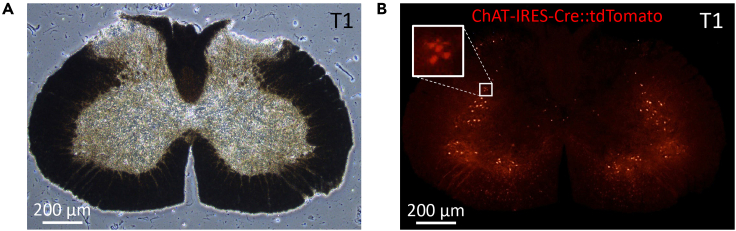
Figure 4.
Spinal cord section at level T1
(A) Brightfield image of spinal cord (10X). Scale bar, 200 μm.
(B) Expression of tdTomato fluorescence in the same section. The rectangles highlight sympathetic preganglionic neurons located within the IML. Scale bar, 200 μm.
Preparation of spinal cord for cryosectioning (for histology experiments)
Timing: 3 days
This step fixes, dehydrates, and embeds the spinal cord tissue prior to cryosectioning. Make sure that the spinal cord lies straight in a Petri dish during these steps.
-
17.
Place trimmed spinal cord prepared in 4% PFA in a 60 mm Petri dish. Make sure the spinal cord lies straight, remove excess PFA from the surface with paper towel, and wait at least 10 min to attach the spinal cord to the Petri dish.
-
18.
Gently pour 4% PFA to fill the Petri dish, and leave the spinal cord submerged at 4°C for 12 h.
Note: Mouse brain is usually post-fixed with 4% PFA for 16–24 hours. However, spinal cords are relatively thin and require less time for post-fixation (e.g., 2 h,4 4 h,5 or 6–8 h6). In this protocol, we chose 12 hours of post-fixation, and performed antigen retrieval for ChAT immunofluorescence staining. The optimal time and the requirement of antigen retrieval needs to be determined case-by-case, and it is recommended to perform pilot experiments under various conditions.
-
19.
Cryoprotection: After 12 h, remove the 4% PFA and fill the Petri dish with PBS-based 30% sucrose solution. Store it at 4°C for 48 h.
-
20.
After 48 h, detach the spinal cord from the Petri dish and remove moisture.
-
21.
Embedding: Cut off the cervical and lumbar ends of the spinal cord using razor blades, leaving ∼1.8 cm spinal cord in the center. To preserve the T1 level of spinal cord, make sure the spinal cord includes the narrowing part of the cervical enlargement. Store the spinal cord in the embedding medium at −80°C.
Pause point: Once the embedded spinal cord is frozen, it can stably be stored at −80°C for up to 6 months.
-
22.
Following the manufacturer’s instruction, collect serial cryosections (40 μm thickness) of the spinal cord in PBS-filled 24-well plates, placing 5 slices per 1 well. Complete sections of one mouse spinal cord require four 24-well plates.
-
23.
Store the plates at 4°C. Troubleshooting 4.
Note: Spinal cord slices stored in PBS should be used for immunofluorescence staining within 3 days after cryosection for good quality. If you need more time before immunofluorescence staining, you can use cryoprotectant solutions instead of PBS. In this case, make sure you wash the sample with PBS before mounting the slices on slide glasses.
Antigen retrieval and immunofluorescence staining (for histology experiments)
Timing: 2 days
In this protocol, the heat-induced antigen retrieval method was used for better visualization of ChAT immunofluorescence.
-
24.
Mount the spinal cord slices on the slide glass, 1 of 5 slices from 1 well, starting from rostral sections and proceeding to caudal sections. Mounting in 4 rows (12 pieces per row) onto a slide glass requires 2 slide glasses to accommodate spinal cord sections from 1 mouse. Fully dry the mounted sections at 15°C–25°C.
Note: Serial mounting of spinal cord slices (from rostral sections to caudal sections) on slide glasses facilitates tracking of the spinal cord levels.
Note: It is helpful to use a small brush for transferring the slices to the slide glass and 23-gauge syringe needles to align slices within the slide glass before drying.
-
25.
Preheat the 10 mM sodium citrate buffer to 60°C using a water bath during slice mounting.
-
26.
Washing: immerse the slide glasses in a PBS-filled jar and place it on the gently working rocker for 5 min. Troubleshooting 5.
-
27.
After one cycle of washing, immerse the slide glasses to a heated 10 mM sodium citrate buffer for 30 min.
-
28.
After 30 min of antigen retrieval, repeat 3 cycles of washing with PBS to remove antigen retrieval buffer.
-
29.
After washing, dry the slide glasses at 15°C–25°C and draw edge lines with liquid blocker pen.
CRITICAL: Drawing the edge lines with liquid blocker pen should not be performed prior to heat-induced antigen retrieval.
Pause point: When the slices are fully dry on the slide glass, you can store the slide glass at –80°C for 5–6 months, before or after heat-induced antigen retrieval.
-
30.
When the liquid blocker is dry, proceed to immunofluorescence staining starting from blocking and permeabilization step as described in Table 1.
Note: Theoretically, 18.2 mm-long spinal cord (T1 to L1)3 would contain 91 x 5 slices when cut in sections with 40 μm thickness. Therefore, we assumed that level T1 has 3.4 slices, and each of levels T2 to L1 has 6.8 slices when mounted on the slide glass. The level T1 was determined as described in Figure 4.
Expected outcomes
In preparation for patch-clamp experiments, acute spinal cord slices will be collected in the lattice-shaped storage chamber in order from level T1 to L1. Given the estimated length between levels T1 and L1 in mice (18.2 mm),3 the 8 × 8 lattice-shaped storage chamber would accommodate 64 slices from one mouse. A representative trace of whole-cell patch-clamp recordings of SPNs (Figure 5A) shows a baseline membrane potential of -46 mV of a spontaneously-firing neuron (Figure 5B).
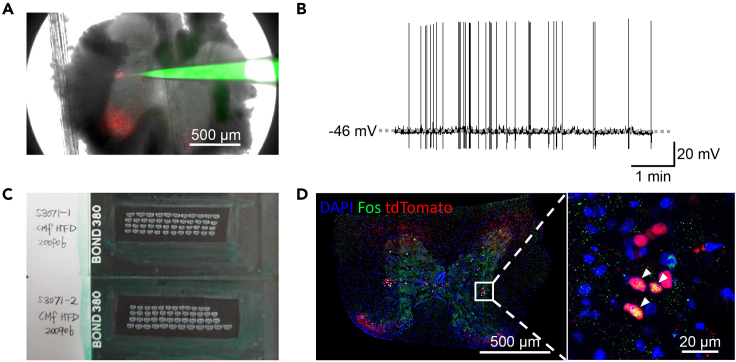
Figure 5.
Expected results
(A) 4X image obtained with a fluorescence microscope attached to a patch-clamp set. Red fluorescence represents tdTomato signal from a ChAT-IRES-Cre::tdTomato reporter mouse. Red fluorescence in the IML represents SPNs, while that in the anterior horn represents α-motor neurons. Green fluorescence represents Alexa 488 added to the pipette solution. Scale bar, 500 μm.
(B) A representative trace of spontaneously firing SPN. Scale bar, 1 min (horizontal); 20 mV (vertical).
(C) Slide glasses after complete immunofluorescence staining of T1-L1 spinal cord from one mouse.
(D) Confocal microscope image of Fos staining from a ChAT-IRES-Cre::tdTomato reporter mouse. Blue fluorescence represents DAPI, green fluorescence represents Fos immunoreactivity, and red fluorescence represents SPNs. The rectangular area in the left image (20×) is enlarged to the right image (40×). Fos expressing ChAT neurons are indicated with arrowheads. Scale bar, 500 μm in left image; 20 μm in right image.
In preparation for histology experiments, collecting the cryosections into four 24-well plates results in 96 slices (1 of 5 slices/well X 24 well/plate X 4 plates) from one mouse (Figure 5C). Immunofluorescence staining for Fos in ChAT-IRES-Cre::tdTomato reporter mice labels of active SPNs (Figure 5C and 5D).
Limitations
Due to the elongated shape of spinal cord, obtaining acute coronal sections results in a lot more slices (64 slices in this protocol) compared to studies of other brain areas (typically 2–5 slices). This makes the step of slice preparation very laborious. However, it is quite difficult to distinguish IML SPNs from other cholinergic neurons in acute slice preparations if we obtain transverse or sagittal spinal cord slices. Therefore, while laborious, it is best to obtain acute spinal cord slices in coronal sections.
Troubleshooting
Problem 1
Perfusate inflates the lung or makes bubbles in the mouth and nose during the transcardial perfusion, [Related to step 3 of spinal cord preparation (for patch-clamp and histology experiments)].
Potential solution
-
•
The tip of the syringe needle should be placed in the left ventricle. If the tip is located in the right atrium, the perfusate will inflate both lungs and regurgitate into the airway, mouth and nose. To avoid this, the other hand (the one not holding the syringe) should grab a forceps to hold the needle in place to minimize the movement of syringe needle during the perfusion.
-
•
If lung inflation or regurgitation occurs during transcardial perfusion, additional perfusion may be necessary until the color of the liver changes from deep brown to beige.
-
•
Perfusion rate is another important point. If the perfusion rate is too high, perfusate may leak or regurgitate out of systemic circulation due to high pressure. We perfused at a rate of about 10–20 mL per minute.
Problem 2
The spinal cord is detached from the agarose block in the buffer tray, [Related to step 14 of making acute spinal cord slices with a vibratome (for patch-clamp experiments)].
Potential solution
-
•
Moisture on the surface of agarose block or spinal cord reduces adhesion power. Remove the moisture before and after placing the spinal cord on the agarose block with tip of paper towel or filter paper.
-
•
Proper amount of glue is critical. If you made a groove on the edge of the agarose block, be careful to avoid overflow of the glue.
-
•
The super glue introduced in this protocol requires 15 s for initial fixation, but it requires 24 h for full fixation. For reliable attachment of the spinal cord to the agarose block, leave them at least for 5 min after applying glue.
-
•
If the spinal cord is detached, remove moisture completely from the cord. Apply glue to a new agarose block, load the spinal cord, and wait at least for 10 min before resuming slice sectioning.
Problem 3
Cells are not good enough for patch-clamp experiments, [Related to step 16 of making acute spinal cord slices with a vibratome (for patch-clamp experiments)].
Potential solution
-
•
Try to minimize the time from perfusion to vibratome sectioning.
-
•
The modified ACSF solution should be ice-cold throughout vibratome sectioning. Put ice or dry ice around the buffer tray to maintain the low temperature.
-
•
After vibratome sectioning, the sections should immediately be transferred to 34°C storage solution and be given at least 1 h of recovery time.
-
•
Check the osmolarity of ACSF solutions. The osmolarity of well-made ACSF solutions for storage and recording is around 290 mOsm/L.
Problem 4
Spinal cord slices are not intact when immersed in PBS after cryosectioning, [Related to step 23 of preparation of spinal cord for cryosectioning (for histology experiments)].
Potential solution
-
•
The embedding mold is extremely hard when it is right out of storage at –80°C. Cryosectioning in this condition is likely to damage spinal cord samples and may result in broken sections when they are immersed in PBS.
-
•
To minimize tissue damage, it is helpful to place the sample at –20°C at least 1 h before cryosectioning.
-
•
High sectioning speed may also damage the sample. We obtained cryosections at a rate of less than 1 section per second.
Problem 5
Spinal cord slices are detached from the slide glass during washing, [Related to step 26 of antigen retrieval and immunofluorescence staining (for histology experiments)].
Potential solution
-
•
Confirm the slide glass is an adhesive one.
-
•
Over-drying can lead to easy detachment of the slices. Because drying time differs between the edge and center slices, supply adequate amount of PBS to avoid over drying slices at the edge of slides.
-
•
Be gentle in adding PBS during the washing step.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, J.-W.S. (jwsohn@kaist.ac.kr).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This work was supported by grants from the National Research Foundation of Korea (NRF-2020M3A9D8039920 and NRF-2022R1A2C3005613 to J.-W.S.).
Author contributions
S.-H.J. and J.-W.S. wrote and edited the article.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2023.102345.
Contributor Information
Sang-Hyeon Ju, Email: jshsignal@cnuh.co.kr.
Jong-Woo Sohn, Email: jwsohn@kaist.ac.kr.
Data and code availability
Data is available upon request.
References
- 1.Ju S.H., Yun H., Oh Y., Choi Y., Sohn J.-W. Melanocortin-4 receptors activate sympathetic preganglionic neurons and elevate blood pressure via TRPV1. Cell Rep. 2022;41:111579. doi: 10.1016/j.celrep.2022.111579. [DOI] [PubMed] [Google Scholar]
- 2.Sohn J.-W., Harris L.E., Berglund E.D., Liu T., Vong L., Lowell B.B., Balthasar N., Williams K.W., Elmquist J.K. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152:612–619. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison M., O’Brien A., Adams L., Cowin G., Ruitenberg M.J., Sengul G., Watson C. Vertebral landmarks for the identification of spinal cord segments in the mouse. Neuroimage. 2013;68:22–29. doi: 10.1016/j.neuroimage.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 4.Paramos-de-Carvalho D., Martins I., Cristóvão A.M., Dias A.F., Neves-Silva D., Pereira T., Chapela D., Farinho A., Jacinto A., Saúde L. Targeting senescent cells improves functional recovery after spinal cord injury. Cell Rep. 2021;36:109334. doi: 10.1016/j.celrep.2021.109334. [DOI] [PubMed] [Google Scholar]
- 5.Nelson T.S., Fu W., Donahue R.R., Corder G.F., Hökfelt T., Wiley R.G., Taylor B.K. Facilitation of neuropathic pain by the NPY Y1 receptor-expressing subpopulation of excitatory interneurons in the dorsal horn. Sci. Rep. 2019;9:7248–7314. doi: 10.1038/s41598-019-43493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding L., Chu W., Xia Y., Li T., Shi M., Zhou F.-Q., Deng D.Y. UCHL1 inhibited by A1 astrocytes facilitates aggregates clearance to promote neural stem cells activation after spinal cord injury. bioRxiv. 2021 doi: 10.1101/2021.09.23.461600. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon request.