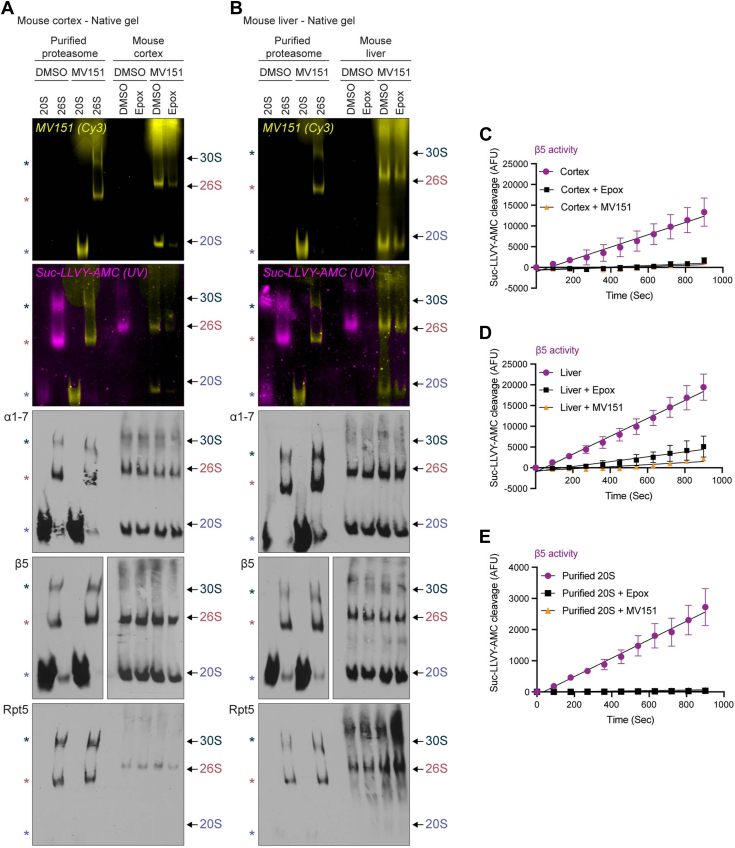
Figure 3.
Activity of doubly-capped (30S), singly-capped (26S), and uncapped (20S) proteasome complexes are more efficiently measured with MV151 than AMC fluorescent substrate.A, top, purified human 20S/26S proteasomes or lysates from mouse cortical tissue were incubated with vehicle (DMSO) or MV151. Each cortical sample was also incubated with vehicle (DMSO) or the proteasome inhibitor epoxomicin (Epox). To determine the relative activity of distinct proteasome complexes, samples were separated using 4% native-PAGE. First, native gels were imaged with the Cy3 channel to detect MV151 incorporation (yellow). Second, native gels were treated with Suc-LLVY-AMC (AMC) and imaged under UV light to monitor proteasome chymotrypsin-like activity (purple). Bottom, imaged gels were then immunoblotted with antibodies to the indicated core proteasome subunits (α1–7, β2, β5) or a 19S regulatory particle subunit (Rpt5/S6). Cy3, UV, and immunoblot bands correspond to individual proteasome complexes (30S, 26S, 20S) (asterisks for purified proteasomes and arrows for tissue homogenate). B, top, in-gel proteasome activity assays with purified human 20S/26S proteasomes or lysates from mouse liver tissue were performed as in (A). Bottom, representative immunoblots of the native gels. C–E, Mouse cortex lysates (C), liver lysates (D), or purified human 20S proteasome (E) were treated in a microplate with vehicle (DMSO), MV151, or epoxomicin (Epox), and incubated with Suc-LLVY-AMC. Fluorescence intensity was measured every 1.5 min for 10 min using a microplate fluorescence reader. Data are represented as mean ± SEM (n = 3). See also Fig. S3.