Abstract
Dry eye disease (DED) is a commonly occurring, multifactorial disease characterized by reduced tear film stability and hyperosmolarity at the ocular surface, leading to discomfort and visual compromise. DED is driven by chronic inflammation and its pathogenesis involves multiple ocular surface structures such as the cornea, conjunctiva, lacrimal glands, and meibomian glands. The tear film secretion and its composition are regulated by the ocular surface in orchestration with the environment and bodily cues. Thus, any dysregulation in ocular surface homeostasis causes an increase in tear break-up time (TBUT), osmolarity changes, and reduction in tear film volume, all of which are indicators of DED. Tear film abnormalities are perpetuated by underlying inflammatory signaling and secretion of inflammatory factors, leading to the recruitment of immune cells and clinical pathology. Tear-soluble factors such as cytokines and chemokines are the best surrogate markers of disease severity and can also drive the altered profile of ocular surface cells contributing to the disease. Soluble factors can thus help in disease classification and planning treatment strategies. Our analysis suggests increased levels of cytokines namely interleukin-1β (IL-1β), IL-2, IL-4, IL-6, IL-9, IL-12, IL-17A, interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α); chemokines (CCL2, CCL3, CCL4, CXCL8); MMP-9, FGF, VEGF-A; soluble receptors (sICAM-1, sTNFR1), neurotrophic factors (NGF, substance P, serotonin) and IL1RA and reduced levels of IL-7, IL-17F, CXCL1, CXCL10, EGF and lactoferrin in DED. Due to the non-invasive sample collection and ease of quantitively measuring soluble factors, tears are one of the best-studied biological samples to molecularly stratify DED patients and monitor their response to therapy. In this review, we evaluate and summarize the soluble factors profiles in DED patients from the studies conducted over the past decade and across various patient groups and etiologies. The use of biomarker testing in clinical settings will aid in the advancement of personalized medicine and represents the next step in managing DED.
Keywords: Biomarker, chemokines, cytokines, dry eye disease, growth factors, tear-soluble factors
Dry eye disease (DED) is recognized as a multifactorial disease, with impaired homeostasis of the tear film as the central key player in disease pathophysiology.[1] Broad ocular symptoms of DED include discomfort or pain, visual disturbance, tear hyperosmolarity, tear film instability, and chronic inflammation contributed by any of the ocular surface structures such as the cornea, conjunctiva, lacrimal glands, and meibomian glands.[2] DED is one of the most commonly occurring ocular surface disorders with an estimated prevalence ranging from 3.8% to 64% with an overall prevalence of 20.1% in Asian populations.[3] Data from hospitals on its incidence clearly show that occupation, socio-economic status, urban residence, age, and sex are the major risk factors for DED in the Indian population.[4] Recent studies have reported the prevalence of DED at 32% with the majority of the severe DED cases falling within the age of 21–40 years.[5] Considering the large prevalence of DED in the working population and its persistent negative effect on the quality of life, it remains a major public health concern.[6]
DED is a chronic inflammatory condition that is contributed by multiple factors such as environment, systemic and ocular allergies, aging, autoimmunity, smoking, and contact lens use, which promote tear film instability.[7] Tear Film and Ocular Surface Society, Dry Eye WorkShop II (TFOS DEWS II) consensus upon the vicious circle of inflammation-driven tear film abnormalities.[1] These abnormalities are often driven by underlying stress-mediated inflammatory signaling, which further triggers the secretion of pro-inflammatory factors and the recruitment of immune cells.[8]
In this review, we focus on various types of DED and associated tear molecular factors that were reported in the last decade. Since dysregulation of immune/inflammation regulatory pathways is one of the key driving factors of DED, we synthesize the various observations in a systematic manner to evaluate the contribution of various soluble factors to the overall inflammatory profile. Recent studies in understanding the role of inflammation in the pathogenesis of DED have led to the recognition that altered immune factor regulation and secretion, leading to heightened immune responses is the primary driver of DED pathology.[8-10] It has also led to the identification of specific inflammatory factors, which can act as biomarkers for DED[11-13] as well as targets for appropriate management of the progression of ocular surface damage.[2,14,15] Because different studies use different approaches and represent different ethnic groups and etiologies, it is imperative to collate recent studies pertaining to the measurement of soluble factor levels in tears of dry eye patients as it is important not only to understand and reach consensus on key molecular factors driving the disease but also to identify plausible biomarkers for point of care kit development for use in clinics. This study thus focuses on discussing the molecular factors that have been reported in various studies in the last decade and understanding the significance of these factors in disease pathophysiology.
Methods
A search in PubMed for articles published between 2012 and before September 2022 was performed with the keywords “tear,” “soluble factors,” “Dry eye disease,” and their combinations. Filters for “10 years,” species as “humans” and language as “English” were kept for the articles included in the present review. A total of 534 articles were obtained. The studies using in vitro and in vivo models and not specifically using tear samples from the DED subjects were excluded. One hundred two papers were included in the final analysis. Ninety-five major factors were uniquely identified and the findings are reported in Table 1. To calculate the Importance score, as shown in Fig. 1a, weightage was assigned based on the status of soluble factors reported in the articles. All soluble factors reported as decreasing significantly were given a score of −2, −1 for decreased, 0.5 for no change, +1 for increase, and +2 for increase significantly. These status scores were multiplied by their respective number of publications. The overall sum of these parameters determines the Importance score for each soluble factor.
Table 1.
Number of factors in each major class that was measured in the tear fluid of DED patients
| Cytokines | 26 |
| Chemokines | 12 |
| Growth factors | 6 |
| Colony stimulating factor | 1 |
| Enzymes | 15 |
| Soluble cell adhesion molecule/soluble receptors | 9 |
| Neurotrophic factors/Neuropeptide | 7 |
| Mucins | 2 |
| Heat shock proteins | 4 |
| Others | 13 |
| Total | 95 |
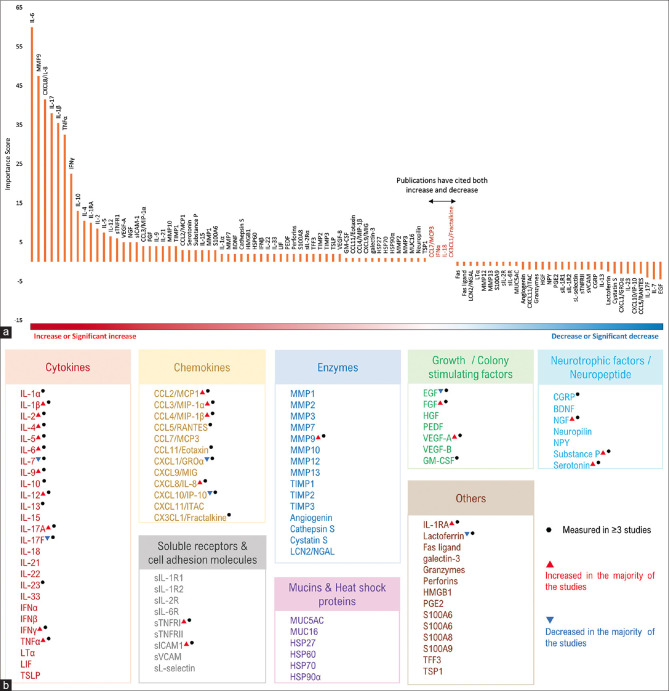
Figure 1.
(a) Overview of tear-soluble factor status in DED patients as reported across various publications in the last decade. Y-axis represents the Importance score, which shows the prominence of soluble factors identified within the articles used in this study. X-axis lists the biomarkers in the decreasing order of Importance score (the gradient color bar has a red color, which indicates a significant increase or increase; white for no change in the status; and blue for a decrease or significant decrease). (b) List of tear-soluble factors identified under each major class in tears of DED patients. Factors that were reported to be measured in at least three publications are indicated by a black dot. Factors that were observed to significantly increase in three publications or more are indicated by a red triangle. Factors that were observed to significantly decrease in three publications or more are indicated by a blue inverted triangle
Tear-Soluble Factors in DED
Multiple cytokines, chemokines, growth factors, colony-stimulating factors, enzymes, soluble receptors, neuropeptides, mucins, heat shock proteins, and others have been reported in association with DED patients. The pro-inflammatory milieu drives the trafficking and infiltration of immune cells to the ocular surface, leading to barrier disruption, neural sensitization, and glandular secretory dysfunction.[16-18] Classically, DEDs largely have increased evaporation of tears, namely, EDED, and one with reduced tear production as ADED.[19] Ocular surface immunity involves both innate immunity and adaptive immunity components.[20] Innate immune response on the ocular surface is triggered and maintained by the corneal epithelium. At the ocular surface natural killer (NK) cells, macrophages, dendritic cells, and antigen-presenting cells (APCs) contribute to innate immunity and differentiated T cells contribute to adaptive immunity responses.[21] Significantly higher proportions of leukocytes, neutrophils, CD4 and CD8 T cells have been reported in both ADED and EDED patients.[22]
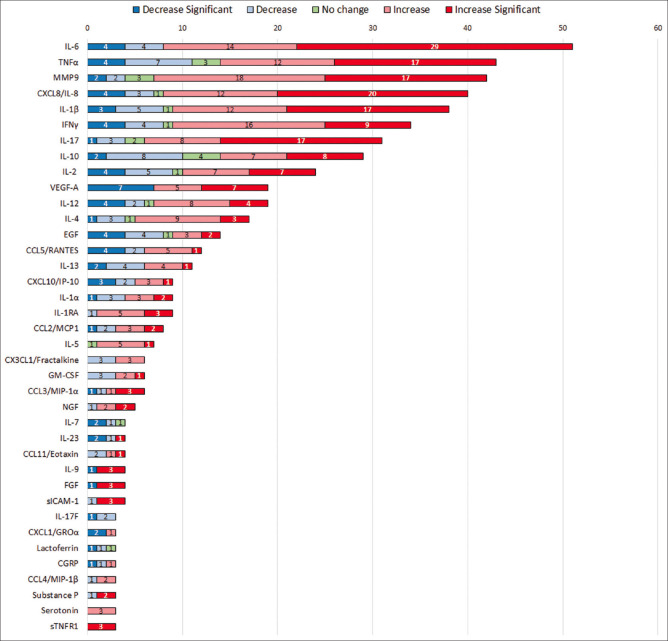
A total of 95 soluble factors were reported under different major subclasses of soluble factors in the tears of DED subjects as listed in Table 1 and Fig. 1b. The overall status, that is, increased or decreased levels of these soluble factors reported in DED tear samples are represented in Fig. 1a. Further, 38 top hit analytes having an increased or decreased status as reported in more than or at least in three publications are shown in Fig. 2. Although most pro-inflammatory, proapoptotic factors are upregulated, a few studies also report them to be reduced, this might be due to sampling and measurement factors as well as cohort characteristics.
Figure 2.
Bar plot of 38 top hit (≥3 publications with consistent report) soluble factors identified in DED. Differential levels of soluble factors in tears of DED subjects. Numbers inside colored bars indicate the number of publications for each analyte. A dark blue color box indicates significantly decreased levels of analytes, light blue indicates decreased levels of analytes, green indicates no change, light pink indicates increased levels, and red corresponds to significantly increased levels of analytes as reported in the last 10 years across publications
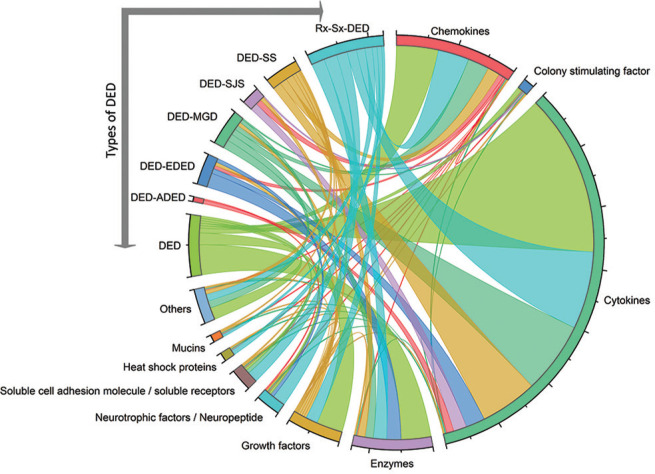
We have classified the DED based on the publications included in the present study. In addition, triaging questions and ancillary testing in concordance with clinical signs are incorporated for differentiating DED from other ocular surface diseases.[23] Seven categories of DED and top hit analytes levels are listed in Table 2 and Fig. 3. However, clinical subtypes such as meibomian gland dysfunction (DED-MGD), systemic autoimmune diseases such as Sjögren’s syndrome (DED-SS), Steven–Johnson syndrome (DED-SJS) and treatment- or surgery-induced secondary dry eye disease (Rx-Sx-DED) are based on symptoms. Interestingly, our group has shown different ocular immune surface profiles unique to EDED and ADED.[22] This prompted us to investigate the distribution of analytes with their respective molecular status in the tears of various types of DED subjects are illustrated in Fig. 4.
Table 2.
Type of tear-soluble factors and their status in dry eye disease in the last 10 years
| Analyte | Status | Reference |
|---|---|---|
| Chemokines | ||
| Fractalkine/CX3CL1 | 3/6 (up) | [8,24,25] |
| 3/6 (down) | [8,26,27] | |
| IL-8/CXCL8 | 12/40 (up) | [9,11,25-34] |
| 20/40 (up*) | [8,13,15,16,24,35-45] | |
| 3/40 (down) | [11,34,46] | |
| 4/40 (down*) | [14,47-49] | |
| 1 (No change) | [50] | |
| IP-10/CXCL10 | 3/9 (up) | [13,24,28] |
| 1/9 (up*) | [51] | |
| 2/9 (down) | [26,46] | |
| 3/9 (down*) | [36,40,52] | |
| MCP-1/CCL2 | 3/8 (up) | [8,27,33] |
| 2/8 (up*) | [39,44] | |
| 2/8(down) | [13,29] | |
| 1/8(down*) | [48] | |
| MIP-1α/CCL3 | 1/6 (up) | [43] |
| 3/6 (up*) | [8,43,53] | |
| 1/6 (down) | [33] | |
| 1/6 (down*) | [8] | |
| RANTES/CCL5 | 5/12 (up) | [8,25,26,33,52] |
| 1/12(up*) | [29] | |
| 2/12 (down) | [8,40] | |
| 4/12 (down*) | [13,39,47,54] | |
| Cytokines | ||
| GM-CSF | 2/6 (up) | [50,55] |
| 1/6 (up*) | [39] | |
| 3/6 (down) | [9,32,33] | |
| IFN-γ | 16/34 (up) | [9,10,28-30,32,40,43,50,52,56-60] |
| 9/34 (up*) | [8,16,57,31,43,44,61-63] | |
| 4/34 (down) | [33,38,46] | |
| 4/34 (down*) | [13,37,39,64] | |
| 1/34 (No change) | [14] | |
| IL-10 | 7/29 (up) | [16,30,33,56,60,63,65] |
| 8/29 (up*) | [8,9,31,32,40,50,58,62] | |
| 8/29 (down) | [8,13,28,52,27,38,46,61] | |
| 2/29 (down*) | [37,39] | |
| 4/29 (No change) | [14,29,38,58] | |
| IL-12 | 5/10 (up) | [9,30,32,33,50] |
| 2/10 (up*) | [62,66] | |
| 1/10 (down) | [13] | |
| 2/10 (down*) | [37,39] | |
| IL-12p70 | 3/9 (up) | [24,30,40] |
| 2/9 (up*) | [43] | |
| 1/9 (down) | [38] | |
| 2/9 (down*) | [38,39] | |
| 1/9 (No change) | [16] | |
| IL-13 | 4/11 (up) | [28,43,57] |
| 1/11 (up*) | [62] | |
| 4/11 (down) | [13,33,43,46] | |
| 2/11 (down*) | [39,47] | |
| Il-17 | 1/11 (up) | [33] |
| 9/11 (up*) | [57,58,63,67-70] | |
| 1/11 (down) | [28] | |
| IL-17A | 7/20 (up) | [29,30,38,56,61,71] |
| 8/20 (up*) | [29,31,39,52,53,59,62,71] | |
| 2/20 (down) | [40,46] | |
| 1/20 (down*) | [17] | |
| 2/20 (No change) | [8,14] | |
| IL-1α | 3/8 (up) | [28,34,43] |
| 2/8 (up*) | [43,47] | |
| 3/8 (down) | [16,29,34] | |
| IL-1β | 12/38 (up) | [8,10,13,16,28,29,33,38,40,43,63,71] |
| 17/38 (up*) | [9,30,31,32,42-44,47,50,52,53,66,67,71-74] | |
| 5/38 (down) | [8,11,29,38,46] | |
| 3/38 (down*) | [48,75,76] | |
| 1/38 (No change) | [14] | |
| IL-2 | 7/24 (up) | [9,28,32,57,58,60,61] |
| 7/24 (up*) | [8,39,47,57,62,63,67] | |
| 5/24 (down) | [8,13,33,40,58] | |
| 4/24 (down*) | [52,37,64,77] | |
| 1/24 (No change) | [50] | |
| IL-4 | 9/17 (up) | [8,9,13,57,33,50,55,60] |
| 3/17 (up*) | [32,58,62] | |
| 3/17 (down) | [28,52,61] | |
| 1/17 (down*) | [37] | |
| 1/17 (No change) | [58] | |
| IL-5 | 5/7 (up) | [9,57,32,33] |
| 1/7 (up*) | [62] | |
| 1/7 (No change) | [50] | |
| IL-6 | 14/51 (up) | [8,10,13,25,28,29,30,33,34,38,40,60,61,65] |
| 29/51 (up*) | [9,15,16,24,26,31,32,35-37,41-45,50,55,56,58,62,63,67,69] | |
| 4/51 (down)# | [27,34,38,46] | |
| 4/51 (down*)# | [14,17,47,64] | |
| IL-9 | 3/4 (up*) | [8,53,62] |
| 1/4 (down*) | [8] | |
| TNF-α | 12/42 (up) | [10,16,28,29,33,34,38,40,59,60,71] |
| 17/42 (up*) | [31,41,43,44,54,55,56,58,63,66,69,71,78] | |
| 7/42 (down) | [8,9,13,32,38,46,61] | |
| 4/42 (down*) | [39,49,64,76] | |
| 2/42 (No change) | [14,50] | |
| IL-1Ra | 5/9 (up) | [8,13,26,25,33] |
| 3/9 (up*) | [24,31,40] | |
| 1/9 (down) | [27] | |
| Enzymes | ||
| MMP-9 | 18/42 (up) | [12,25,27,30,79-91] |
| 17/42 (up*) | [13,24,26,31,36,52,92-99] | |
| 2/42 (down)# | [46,100] | |
| 2/42 (down*)# | [75,77] | |
| 3/42 (No change) | [11,70,101] | |
| Growth factors | ||
| EGF | 3/14 (up) | [8,57,71] |
| 2/14 (up*) | [51,71] | |
| 4/14 (down) | [24,25,31,46] | |
| 4/14 (down*) | [13,26,40,42] | |
| 1/14 (No change) | [27] | |
| FGF | 3/4 (up*) | [36,39,53] |
| 1/4 (down*) | [36] | |
| VEGF | 5/17 (up) | [24,25,26,30,40] |
| 7/17 (up*) | [36,45,55,62,67,102] | |
| 5/17 (down*) | [9,32,39,47,50] | |
| Neurotrophic and neuropeptide factors | ||
| Serotonin | 3/3 (up) | [18,65,81] |
| NGF | 2/5 (up) | [8,11] |
| 2/5 (up*) | [47,72] | |
| 1/5 (down) | [11] | |
| Soluble cell adhesion molecule and soluble receptors | ||
| ICAM-1 | 3/4 (up*) | [15,16,42] |
| 1/4 (down) | [29] | |
*Indicates significant (up or down) reported levels. #Expression post anti-inflammatory treatments
Figure 3.
Chord plot illustrating the relationship between types of DEDs and their associated analytes in major subclasses. DED: Dry eye disease, DED-ADED: Aqueous deficient, DED-EDED: Evaporative, DED-MGD: Meibomian gland dysfunction related dry eye, DED-SJS: Steven–Johnson syndrome-related dry eye, DED-SS: Sjögren’s syndrome associated dry eye, Rx-Sx-DED: Treatment or surgery induced dry eye disease
Figure 4.
Sankey plot illustrating the status of various tear analytes in seven types of DEDs. The thickness of the line indicates the presence of an analyte in the number of publications followed by their status (increase significantly, increase, no change, decrease and decrease significantly) in DED, DED-ADED, DED-EDED, DED-MGD, DED-SJS, DED-SS, Rx-Sx-DED
Cytokines
Most secretory proinflammatory factors have been reported to be over-expressed in DED.[56,57,71] For example, cytokines such as TNFα, IL-6, and IL-1, which are highly expressed in DED are known to stimulate the maturation of APCs. IL-1β, apart from being pro-inflammatory in nature, is also involved in cellular apoptosis and pain hypersensitivity.[8] IL-17, which acts upon different cell types as a pro-inflammatory and antimicrobial cytokine, induces the production of IL-6, both of which are upregulated in DED.[28,67] Other cytokines, which have been reported to be dysregulated in DED include IL-2, IL-4, IL-9, IL-10, and IFN-γ. Cytokines are critical controllers of tissue growth, migration, differentiation, and development and are potential factors, which can serve as biomarkers for DED. Among cytokines, IFN-γ, TNF-α, interleukin-1 receptor antagonist (IL-1RA), and interleukins (IL)-1β, -4, -5, -6, -9, -12, -17, -17A levels were increased in more than 60% of the reported publications [Figs. 1b, 2, Table 2]. Protective factors such as IL-10 are reported to be equally increased and decreased across the reports in the past decade. Fourteen studies showed decreased or no change and 15 studies showed an increase in IL-10 levels [Fig. 2, Table 2]. Based on the chord plot in Fig. 3 and Table 2, tear cytokines are associated with all seven types of DEDs and constitute the major tear-soluble factors reported to date. Thus, this implies the active inflammatory status in DED subjects.
Chemokines
Chemokines are a family of secreted signaling factors, which primarily induce the migration of immune cells. Chemokines such as C-X-C motif chemokine ligand 8 (CXCL8/IL-8), CC-chemokine receptor 5 (CCL5) or RANTES, interferon γ-induced protein (IP-10/CXCL10), monocyte chemoattractant protein-1 (MCP1/CCL2), macrophage inflammatory protein (MIP)-1α/CCL3, and fractalkine/CX3CL1 were reported in dry eye disease.[13,29]Among these, 80% of articles reported an increase in IL-8 levels, whereas MCP-1 and MIP-1α were increased in more than 60% of reported publications. The above mentioned chemokines were found to be highly expressed in DED [Table 2, Figs. 1b, 2]. RANTES induces the migration of mature APCs and leukocytes to the site of inflammation.[26] MCP-1 is required for the migration and infiltration of monocytes. IL-8, a known pro-inflammatory chemokine secreted by mononuclear macrophages, epithelial cells, and fibroblasts, results in fibrosis, neovascularization, and endothelial dysfunction and positively correlates with disease severity of multiple ocular diseases.[15,35,36] Variable levels of tear chemokines were reported in DED, DED- EDED, DED-MGD, DED-SJS, DED-SS, and Rx-Sx-DED [Figs. 3 and 4].
Soluble Receptors and Cell Adhesion Molecules
Increased expression of cell adhesion molecules such as intercellular adhesion molecules (ICAMs) and that of MMPs on corneal epithelium as well as conjunctival cells and cells of lacrimal glands has been reported in patients with DED.[79,92] ICAM levels were reported in four publications, of which three showed a significant increase in DED tears [Fig. 2]. ICAM-LFA1 interaction is crucial for the proliferation and recruitment of immune cells as well as the activation of cytokine production.[103] Soluble TNFR1 (sTNFR1) has also been reported to be increased significantly in more than three studies in the past 10 years [Fig. 2]. TNFR1 mediates the majority of the biological effects of TNFα and is ubiquitously expressed. sTNFR1 is generated upon proteolytic cleavage of TNFR1 by the ADAM family of proteins ([TNF-alpha converting enzyme] TACE) through a process called ectodomain shedding.[104] sTNFR1 reduces the fraction of TNFR1 present on the cell surface necessary for the TNFα-mediated signaling. Consequentially, an increase in sTNFR1 fraction and concomitant reduction in surface TNFα expression should result in the dampening of TNFα-mediated response. However, it is not the case in DED as we observe both TNFR1 and sTNFR1 to be at higher levels, which drives apoptosis and inflammation. Both corneal epithelium and stromal fibroblasts are known to express sTNFR1 as well as TACE. Although sTNFR1 levels are used as a biomarker in acute inflammatory conditions[105] and an indication of apoptosis induction,[106] its role in DED is yet to be understood in detail. Dysregulated levels of soluble factors were reported in DED, DED-MGD, DED-SS, and Rx-Sx-DED patients’ tears [Figs. 3 and 4].
Enzymes
ECM-modulating enzymes such as matrix metalloproteases (MMPs) and tissue inhibitors of metalloproteases (TIMPs) have been well characterized with respect to their role in DED.[79,80] Increased levels of MMPs, driven by inflammation are an indication of excessive ECM remodulation, which leads to epithelial cell loss,[24,30,52]which eventually leads to a loss of epithelial layer integrity. MMP9 has not only been suggested to be the biomarker for DED but MMP9 levels along with tear osmolarity have been found to be indicative of disease severity.[79] MMP9 levels were reported in 83% of research articles of which 35 publications reported increased levels and were considered as one of the most reliable markers of DED diagnosis [Table 2, Fig. 2]. Enzymes levels in tears of DED, DED-EDED, DED-MGD, DED-SS, and Rx-Sx-DED subjects were reported in last decade [Figs. 3 and 4].
Growth Factors and Colony-Stimulating Factors
Epidermal growth factor (EGF), FGF, VEGF-A, and GM-CSF are growth factors or colony-stimulating factors, which have been reported to be altered in DED in more than three studies in the past decade. Interestingly, EGF level was reduced in tears of DED subjects [Fig. 2]. EGF and FGF are growth factors known to induce differentiation and proliferation of cells, thereby helping in wound healing and maintaining tissue homeostasis. Reduction in EGF is indicative of goblet cell dysfunction and reduced wound healing capacity of epithelial cells.[107] Animal studies have shown that inhibiting EGFR using erlotinib causes a reduced number of goblet cells as well as reduced secretion of mucins by goblet cells resulting in DED.[108] Chronic inflammatory insult to the ocular surface leads to the invasion of blood and lymphatic vessels into the cornea. It not only facilitates ocular surface inflammation but also cellular trafficking in dry eye disease. Growth factors such as VEGF and FGF, which are elevated in DED promote corneal lymphangiogenesis.[109] Growth factors in tears of DED, DED-EDED, DED-MGD, DED-SJS, DED-SS, and Rx-Sx-DED patients have been seen [Figs. 3 and 4]. Colony-stimulating factors (CSF) are glycoproteins and consist of a small family including granulocyte–macrophage–colony-stimulating factor (GM-CSF), granulocyte–colony-stimulating factor (G-CSF), multiple-colony-stimulating factor, or interleukin 3 (IL-3), and macrophage-colony-stimulating factor (M-CSF). GM-CSF is primarily produced by T cells, activated fibroblasts, and endothelial cells. Under inflammatory stimulation, GM-CSF secretion aids the induction of monocytes/macrophages to promote DED.[110] Animal models of DED suggest similar expression of GM-CSF in the cornea and increased levels in the conjunctiva of DED mice compared to controls.[110] Out of six publications, three stated increased levels of GM-CSF in tears of DED subjects, whereas three showed reduced levels [Table 2, Fig. 2]. Moreover, GM-CSF in also observed in the tears of DED, DED-MGD, and DED-SJS patients [Figs. 3 and 4].
Neurotrophic Factors and Neuropeptides
The cornea is innervated with dense sensory fibers; the fibers terminate into free nerve endings in a tightly packed epithelial layer. Due to the high density of sensory receptors in the cornea, any injury or insult to the cornea is accompanied by pain and hypersensitivity, leading to ocular surface discomfort in diseases such as DED.[52] Neuropeptides and neurotrophic factors play an important role in mediating sensory information and in regulating certain aspects of cell survival and the function of neurons. Serotonin, calcitonin gene-related peptide (CGRP), nerve growth factor (NGF), and substance P are well-known factors studied in DED.[111] Serotonin, a known peripheral nerve sensitizer, has been shown to be higher in tears of DED patients and the levels strongly correlate with symptoms of DED patients.[65] Serotonin, known to be activated by inflammation, might play a role in inducing corneal hypersensitivity in DED.[81] Increased NGF has been shown to play a protective role in DED by improving tear secretion and increasing epithelial cell layer integrity, whereas reduced CGRP is reported to be associated with lacrimal gland dysfunction.[11] Four out of five publications showed increased NGF levels [Table 2 and Fig. 2]. It can be speculated that the observed increase is due to a reparative and rescue response toward ocular surface injury. DED, DED-ADED, DED-EDED, and Rx-Sx-DED patient tears have majorly reported increased neurotrophic factors and peptides [Figs. 3 and 4].
Heat Shock Proteins and Mucins
Heat shock proteins (HSP) are molecular chaperones, which help in protein re-folding, maturation, and degradation. HSPs are present in all the corneal cells, which confer to protect and restore cellular viability during oxidative stress or thermal challenge. The protective role of HSPs is studied in glaucoma, cataract, and cancers. HSP-27, HSP-60, HSP-70, and HSP-90 are present in the cornea.[112] These HSPs were also observed in tears of SS-DED and Rx-Sx-DED patients [Figs. 3 and 4]. The mucin family constitutes secreted gel-forming mucins and membrane-tethered mucins. Mucins are tandem repeats of serine and threonine glycoproteins consisting of carbohydrates. Ocular mucins are the major constituent of the tear film, which provide lubrication and minimize friction during ocular movements and blinking.[108] MUC5AC and MUC16 levels were reported in tears of DED patients [Fig. 1]. Significant reduction of mucins in tears of SS-DED subjects were reported [Fig. 4]. In addition, tears of Rx-Sx-DED subjects have reported mucin levels [Fig. 3].
Overall, the data suggests increased levels of cytokines, namely, IL-1β, IL-2, IL-4, IL-6, IL-9, IL-12, IL-17A, IFN-γ, TNF-α; chemokines (CCL2, CCL3, CCL4, CXCL8); MMP-9, FGF, VEGF-A; soluble receptors (sICAM-1, sTNFR1), neurotrophic factors (NGF, substance P, serotonin) and IL1RA are the most explored and reliable molecules indicative of the DEDs. Reduced levels of IL-7, IL-17F, CXCL1, CXCL10, EGF, and lactoferrin are also commonly reported in DEDs tears [Fig. 5].
Figure 5.
A schematic summary of the most studied soluble factors in DED over the last 10 years. The red arrow indicates that the listed factors have higher levels in the tears of DED subjects, whereas the blue arrow indicates reduced levels of those factors in the disease condition
Conclusion
The ocular surface is constantly exposed to external environment agents and the cells of the ocular surface need to proactively participate in tissue repair and maintenance. Secreted molecular factors are cellular responses towards either external injury/insult or a consequence of internal chronic inflammation where they act in an autocrine and paracrine manner to help maintain tissue homeostasis, aiding cell migration and differentiation. Dysregulated expression of secreted factors is an indication of loss of tissue homeostasis and basic cellular pathways gone amiss. Therefore, it is possible to evaluate tissue status, and as a consequence tissue health by measuring these biomarkers in the tears. Secreted factors can be thus used as potential biomarkers and indicators of not only disease severity but also to understand major cell types contributing to disease pathology. The underlying causes of DED are complex, making it necessary to create a personalized treatment plan for each patient rather than using a one-size-fits-all approach or promoting a single medication or therapy as a cure-all. Therefore, a multi-marker tear protein profile can be used to tailor treatment options for patients, such as selecting the most effective combination of medical therapies (such as anti-inflammatory molecules) and interventional therapies (such as IPL and VP). Hence, we anticipate that point-of-care biomarker diagnostic tests will be made available to clinics shortly.
Future Perspectives
The factors identified are strongly associated with disease severity and can help in planning better treatment strategies in a patient-specific manner, paving way for a personalized medicine approach in managing DED, particularly in iatrogenic cases such as post-refractive surgery or cataract surgery. Hence, the next step is to develop a reliable, point-of-care diagnostic test for accurate and rapid measurement of key molecular factors associated with DED pathobiology. The current review has shown that the key factors such as MMP9, IL-6, TNFα, IL-1β, IL-17A, sICAM1, and IL-10 are significantly altered, albeit at different levels across various types of DED subjects in the last decade. It is well known that many patients present to the cornea clinic with symptoms but without signs, thus necessitating the use of inflammatory biomarker analysis to assist the ophthalmologist to plan potential treatments. In addition, many subjects that do not have symptoms may have signs, which will need to be addressed before surgical procedures. In all these cases, biomarker profiles can be greatly useful for patient stratification. Hence, we are developing a point-of-care diagnostic kit Bio-M Pathfinder (NovoMol-Dx, India, a customized version of the Ella™ Automated ELISA system, Bio-Techne®Corporation, Minnesota, USA) for clinical use. Such a test will not only identify the molecular status in DED patients but also help define the risk of DED development in clinically healthy eyes undergoing invasive procedures such that they may be managed appropriately. In addition, knowledge of the particular biomarker(s) altered in a subject can help a clinician decide, which drug to use to treat them, given the multitude of DED treatment options available today. Therefore, biomarker testing in DED can usher in a new era of “personalized treatment” for DED.
Financial support and sponsorship
This work was supported by Narayana Nethralaya Foundation, Bengaluru, India.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, M VJ, Qu Y, He X, Ou S, Bu J, et al. Dry eye management:Targeting the ocular surface microenvironment. Int J Mol Sci. 2017;18:1398. doi: 10.3390/ijms18071398. doi:10.3390/ijms18071398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai Y, Wei J, Zhou J, Zou W. Prevalence and incidence of dry eye disease in Asia:A systematic review and meta-analysis. Ophthalmic Res. 2022;65:647–58. doi: 10.1159/000525696. [DOI] [PubMed] [Google Scholar]
- 4.Donthineni PR, Kammari P, Shanbhag SS, Singh V, Das AV, Basu S. Incidence, demographics, types and risk factors of dry eye disease in India:Electronic medical records driven big data analytics report I. Ocul Surf. 2019;17:250–6. doi: 10.1016/j.jtos.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Titiyal JS, Falera RC, Kaur M, Sharma V, Sharma N. Prevalence and risk factors of dry eye disease in North India:Ocular surface disease index-based cross-sectional hospital study. Indian J Ophthalmol. 2018;66:207–11. doi: 10.4103/ijo.IJO_698_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benitez-Del-Castillo J, Labetoulle M, Baudouin C, Rolando M, Akova YA, Aragona P, et al. Visual acuity and quality of life in dry eye disease:Proceedings of the OCEAN group meeting. Ocul Surf. 2017;15:169–78. doi: 10.1016/j.jtos.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112:71–81. doi: 10.3238/arztebl.2015.0071. quiz 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco-Vazquez M, Vazquez A, Fernandez I, Novo-Diez A, Martinez-Plaza E, Garcia-Vazquez C, et al. Inflammation-related molecules in tears of patients with chronic ocular pain and dry eye disease. Exp Eye Res. 2022;219:109057. doi: 10.1016/j.exer.2022.109057. doi: 10.1016/j.exer. 2022.109057. [DOI] [PubMed] [Google Scholar]
- 9.Benitez-Del-Castillo Sanchez J, Morillo-Rojas MD, Galbis-Estrada C, Pinazo-Duran MD. Determination of inmune response and inflammation mediators in tears:Changes in dry eye and glaucoma as compared to healthy controls. Arch Soc Esp Oftalmol. 2017;92:210–7. doi: 10.1016/j.oftal.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Wei Y, Gadaria-Rathod N, Epstein S, Asbell P. Tear cytokine profile as a noninvasive biomarker of inflammation for ocular surface diseases:Standard operating procedures. Invest Ophthalmol Vis Sci. 2013;54:8327–36. doi: 10.1167/iovs.13-12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai T, Alwees M, Rost A, Theile J, Dick HB, Joachim SC, et al. Changes of subjective symptoms and tear film biomarkers following femto-LASIK. Int J Mol Sci. 2022;23:7512. doi: 10.3390/ijms23147512. doi:10.3390/ijms23147512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benitez-Del-Castillo JM, Soria J, Acera A, Munoz AM, Rodriguez S, Suarez T. Quantification of a panel for dry-eye protein biomarkers in tears:A comparative pilot study using standard ELISA and customized microarrays. Mol Vis. 2021;27:243–61. [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto-Fraga J, Enriquez-de-Salamanca A, Calonge M, Gonzalez-Garcia MJ, Lopez-Miguel A, Lopez-de la Rosa A, et al. Severity, therapeutic, and activity tear biomarkers in dry eye disease:An analysis from a phase III clinical trial. Ocul Surf. 2018;16:368–76. doi: 10.1016/j.jtos.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Roy NS, Yu Y, Ying GS, Maguire MG, Asbell PA, Group DS. Effect of omega-3 on HLA-DR expression by conjunctival cells and tear cytokine concentrations in the dry eye assessment and management study. Eye Contact Lens. 2022;48:384–90. doi: 10.1097/ICL.0000000000000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Ma Y, Chen X, He S, Lin X, Yu X, et al. Efficacy of bandage contact lens for the management of dry eye disease after cataract surgery. Int Ophthalmol. 2021;41:1403–13. doi: 10.1007/s10792-021-01692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, Chen X, Ma Y, Lin X, Yu X, He S, et al. Analysis of tear inflammatory molecules and clinical correlations in evaporative dry eye disease caused by meibomian gland dysfunction. Int Ophthalmol. 2020;40:3049–58. doi: 10.1007/s10792-020-01489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu R, Rong B, Tu P, Tang Y, Song W, Toyos R, et al. Analysis of cytokine levels in tears and clinical correlations after intense pulsed light treating meibomian gland dysfunction. Am J Ophthalmol. 2017;183:81–90. doi: 10.1016/j.ajo.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Chhadva P, Lee T, Sarantopoulos CD, Hackam AS, McClellan AL, Felix ER, et al. Human tear serotonin levels correlate with symptoms and signs of dry eye. Ophthalmology. 2015;122:1675–80. doi: 10.1016/j.ophtha.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimazaki J. Definition and diagnostic criteria of dry eye disease:Historical overview and future directions. Invest Ophthalmol Vis Sci. 2018;59:DES7–12. doi: 10.1167/iovs.17-23475. [DOI] [PubMed] [Google Scholar]
- 20.Foulsham W, Coco G, Amouzegar A, Chauhan SK, Dana R. When clarity is crucial:Regulating ocular surface immunity. Trends Immunol. 2018;39:288–301. doi: 10.1016/j.it.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez VL, Stern ME, Pflugfelder SC. Inflammatory basis for dry eye disease flares. Exp Eye Res. 2020;201:108294. doi: 10.1016/j.exer.2020.108294. doi: 10.1016/j.exer. 2020.108294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair AP, D'Souza S, Shetty R, Ahuja P, Kundu G, Khamar P, et al. Altered ocular surface immune cell profile in patients with dry eye disease. Ocul Surf. 2021;21:96–106. doi: 10.1016/j.jtos.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15:802–12. doi: 10.1016/j.jtos.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Miguel A, Teson M, Martin-Montanez V, Enriquez-de-Salamanca A, Stern ME, Gonzalez-Garcia MJ, et al. Clinical and molecular inflammatory response in Sjogren syndrome-associated dry eye patients under desiccating stress. Am J Ophthalmol. 2016;161:133–41 e131-2. doi: 10.1016/j.ajo.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez I, Lopez-Miguel A, Enriquez-de-Salamanca A, Teson M, Stern ME, Gonzalez-Garcia MJ, et al. Response profiles to a controlled adverse desiccating environment based on clinical and tear molecule changes. Ocul Surf. 2019;17:502–15. doi: 10.1016/j.jtos.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Teson M, Gonzalez-Garcia MJ, Lopez-Miguel A, Enriquez-de-Salamanca A, Martin-Montanez V, Benito MJ, et al. Influence of a controlled environment simulating an in-flight airplane cabin on dry eye disease. Invest Ophthalmol Vis Sci. 2013;54:2093–9. doi: 10.1167/iovs.12-11361. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-de la Rosa A, Martin-Montanez V, Lopez-Miguel A, Calonge M, Enriquez-de-Salamanca A, Gonzalez-Garcia MJ. Corneal sensitivity and inflammatory biomarkers in contact lens discomfort. Optom Vis Sci. 2016;93:892–900. doi: 10.1097/OPX.0000000000000784. [DOI] [PubMed] [Google Scholar]
- 28.McDonnell PJ, Pflugfelder SC, Stern ME, Hardten DR, Conway T, Villanueva L, et al. Study design and baseline findings from the progression of ocular findings (PROOF) natural history study of dry eye. BMC Ophthalmol. 2017;17:265. doi: 10.1186/s12886-017-0646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Souza S, Shetty R, Nair AP, Agrawal R, Dickman MM, Khamar P, et al. Corneal confocal microscopy features and tear molecular profile in study participants with discordance between ocular surface disease clinical signs and discomfort. J Clin Med. 2022;11:2407. doi: 10.3390/jcm11092407. doi:10.3390/jcm11092407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair S, Vanathi M, Mahapatra M, Seth T, Kaur J, Velpandian T, et al. Tear inflammatory mediators and protein in eyes of post allogenic hematopoeitic stem cell transplant patients. Ocul Surf. 2018;16:352–67. doi: 10.1016/j.jtos.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Chen H, Liang L, Zhong Y, Liang Y, Yu Y, et al. Evaluation of tear protein markers in dry eye disease with different lymphotoxin-alpha expression levels. Am J Ophthalmol. 2020;217:198–211. doi: 10.1016/j.ajo.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Benitez-Del-Castillo J, Cantu-Dibildox J, Sanz-Gonzalez SM, Zanon-Moreno V, Pinazo-Duran MD. Cytokine expression in tears of patients with glaucoma or dry eye disease:A prospective, observational cohort study. Eur J Ophthalmol. 2019;29:437–43. doi: 10.1177/1120672118795399. [DOI] [PubMed] [Google Scholar]
- 33.Yucekul B, Mocan MC, Kocabeyoglu S, Tan C, Irkec M. Evaluation of long-term silicone hydrogel use on ocular surface inflammation and tear function in patients with and without meibomian gland dysfunction. Eye Contact Lens. 2019;45:61–6. doi: 10.1097/ICL.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 34.Villani E, Galimberti D, Del Papa N, Nucci P, Ratiglia R. Inflammation in dry eye associated with rheumatoid arthritis:Cytokine and in vivo confocal microscopy study. Innate Immun. 2013;19:420–7. doi: 10.1177/1753425912471692. [DOI] [PubMed] [Google Scholar]
- 35.Eom HD, Jung JU, Lee KP, Kim J, Yoon DH, Kim MJ, et al. Simplified classification of tear film break-up patterns and their clinicopathological correlations in patients with dry eye disease. Eye Contact Lens. 2021;47:15–9. doi: 10.1097/ICL.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 36.Liu C, Song Y, Wang X, Lai Z, Li C, Wan P, et al. The key role of vegf in the cross talk between pterygium and dry eye and its clinical significance. Ophthalmic Res. 2020;63:320–31. doi: 10.1159/000503636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei Y, Asbell PA. sPLA2-IIa participates in ocular surface inflammation in humans with dry eye disease. Exp Eye Res. 2020;201:108209. doi: 10.1016/j.exer.2020.108209. doi: 10.1016/j.exer. 2020.108209. [DOI] [PubMed] [Google Scholar]
- 38.Akpek EK, Wu HY, Karakus S, Zhang Q, Masli S. Differential diagnosis of sjogren versus non-sjogren dry eye through tear film biomarkers. Cornea. 2020;39:991–7. doi: 10.1097/ICO.0000000000002299. [DOI] [PubMed] [Google Scholar]
- 39.Gurumurthy S, Iyer G, Srinivasan B, Agarwal S, Angayarkanni N. Ocular surface cytokine profile in chronic Stevens-Johnson syndrome and its response to mucous membrane grafting for lid margin keratinisation. Br J Ophthalmol. 2018;102:169–76. doi: 10.1136/bjophthalmol-2017-310373. [DOI] [PubMed] [Google Scholar]
- 40.Cocho L, Fernandez I, Calonge M, Martinez V, Gonzalez-Garcia MJ, Caballero D, et al. Biomarkers in ocular chronic graft versus host disease:Tear cytokine- and chemokine-based predictive model. Invest Ophthalmol Vis Sci. 2016;57:746–58. doi: 10.1167/iovs.15-18615. [DOI] [PubMed] [Google Scholar]
- 41.Peng X, Lu Y, Wei J, Lin T, Lu Q, Liu Q, et al. A cohort study of T helper 17 cell-related cytokine levels in tear samples of systemic lupus erythematosus and Sjogren's syndrome patients with dry eye disease. Clin Exp Rheumatol. 2021;39((Suppl 133)):159–65. doi: 10.55563/clinexprheumatol/tlnr4z. [DOI] [PubMed] [Google Scholar]
- 42.Hu B, Qiu Y, Hong J. Tear cytokine levels in the diagnosis and severity assessment of ocular chronic graft-versus-host disease (GVHD) Ocul Surf. 2020;18:298–304. doi: 10.1016/j.jtos.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Zhao H, Li Q, Ye M, Yu J. Tear luminex analysis in dry eye patients. Med Sci Monit. 2018;24:7595–602. doi: 10.12659/MSM.912010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park Y, Hwang HB, Kim HS. Observation of influence of cataract surgery on the ocular surface. PLoS One. 2016;11:e0152460. doi: 10.1371/journal.pone.0152460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uthaithammarat L, Kasetsuwan N, Chongpison Y, Kasetsuwan P, Reinprayoon U, Nilyanimit P, et al. Lack of HPV in pterygium with no evidence of autoinoculation and the role of cytokines in pterygium with dry eye. Sci Rep. 2021;11:2842. doi: 10.1038/s41598-021-82114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu H, Zeng W, Zhao G, Hong J, Feng Y. Response of tear cytokines following intense pulsed light combined with meibomian gland expression for treating meibomian gland dysfunction-related dry eye. Front Endocrinol (Lausanne) 2022;13:973962. doi: 10.3389/fendo.2022.973962. doi:10.3389/fendo.2022.973962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D'Souza S, Vaidya T, Nair AP, Shetty R, Kumar NR, Bisht A, et al. Altered ocular surface health status and tear film immune profile due to prolonged daily mask wear in health care workers. Biomedicines. 2022;10:1160. doi: 10.3390/biomedicines10051160. doi:10.3390/biomedicines10051160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JH, Min K, Kim SK, Kim EK, Kim TI. Inflammatory cytokine and osmolarity changes in the tears of dry eye patients treated with topical 1% methylprednisolone. Yonsei Med J. 2014;55:203–8. doi: 10.3349/ymj.2014.55.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu SQ, Dai Q, Xu JL, Sheng WY, Xu QB, Zhong LY. Combined effect of traditional Chinese and Western medicine on inflammatory factors in patients with diabetes-induced xerophthalmia. Genet Mol Res. 2016;15 doi: 10.4238/gmr15049030. doi:10.4238/gmr15049030. [DOI] [PubMed] [Google Scholar]
- 50.Pinazo-Duran MD, Galbis-Estrada C, Pons-Vazquez S, Cantu-Dibildox J, Marco-Ramirez C, Benitez-del-Castillo J. Effects of a nutraceutical formulation based on the combination of antioxidants and omega-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders. Clin Interv Aging. 2013;8:139–48. doi: 10.2147/CIA.S40640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agrawal R, Balne PK, Veerappan A, Au VB, Lee B, Loo E, et al. A distinct cytokines profile in tear film of dry eye disease (DED) patients with HIV infection. Cytokine. 2016;88:77–84. doi: 10.1016/j.cyto.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 52.Khamar P, Nair AP, Shetty R, Vaidya T, Subramani M, Ponnalagu M, et al. Dysregulated tear fluid nociception-associated factors, corneal dendritic cell density, and vitamin D levels in evaporative dry eye. Invest Ophthalmol Vis Sci. 2019;60:2532–42. doi: 10.1167/iovs.19-26914. [DOI] [PubMed] [Google Scholar]
- 53.Landsend ECS, Utheim OA, Pedersen HR, Aass HCD, Lagali N, Dartt DA, et al. The level of inflammatory tear cytokines is elevated in congenital aniridia and associated with meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2018;59:2197–204. doi: 10.1167/iovs.18-24027. [DOI] [PubMed] [Google Scholar]
- 54.Pietraszkiewicz AA, Payne D, Abraham M, Garced A, Devarasetty KC, Wall M, et al. Ocular surface indicators and biomarkers in chronic ocular graft-versus-host disease:A prospective cohort study. Bone Marrow Transplant. 2021;56:1850–8. doi: 10.1038/s41409-021-01254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galbis-Estrada C, Pinazo-Duran MD, Cantu-Dibildox J, Marco-Ramirez C, Diaz-Llopis M, Benitez-del-Castillo J. Patients undergoing long-term treatment with antihypertensive eye drops responded positively with respect to their ocular surface disorder to oral supplementation with antioxidants and essential fatty acids. Clin Interv Aging. 2013;8:711–9. doi: 10.2147/CIA.S43191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao F, Hong X, Ding F, Huang S, Lian W, Wang H, et al. High level of inflammatory cytokines in the tears:A bridge of patients with concomitant exotropia and dry eye. Oxid Med Cell Longev. 2021;2021:5662550. doi: 10.1155/2021/5662550. doi:10.1155/2021/5662550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meadows JF, Dionne K, Nichols KK. Differential profiling of T-cell cytokines as measured by protein microarray across dry eye subgroups. Cornea. 2016;35:329–35. doi: 10.1097/ICO.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 58.Lee SY, Han SJ, Nam SM, Yoon SC, Ahn JM, Kim TI, et al. Analysis of tear cytokines and clinical correlations in Sjogren syndrome dry eye patients and non-Sjogren syndrome dry eye patients. Am J Ophthalmol. 2013;156:247–53.e241. doi: 10.1016/j.ajo.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Gad A, Vingrys AJ, Wong CY, Jackson DC, Downie LE. Tear film inflammatory cytokine upregulation in contact lens discomfort. Ocul Surf. 2019;17:89–97. doi: 10.1016/j.jtos.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Li M, Yu Y, Yuan Y, Wang P, Hu X, Zhu C, et al. Rete ridges in eyelid margin and inflammatory cytokines in meibomian gland dysfunction associated with dry eye symptom. Curr Eye Res. 2021;46:202–9. doi: 10.1080/02713683.2020.1788102. [DOI] [PubMed] [Google Scholar]
- 61.Jackson DC, Zeng W, Wong CY, Mifsud EJ, Williamson NA, Ang CS, et al. Tear interferon-gamma as a biomarker for evaporative dry eye disease. Invest Ophthalmol Vis Sci. 2016;57:4824–30. doi: 10.1167/iovs.16-19757. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez-Garcia MJ, Murillo GM, Pinto-Fraga J, Garcia N, Fernandez I, Maldonado MJ, et al. Clinical and tear cytokine profiles after advanced surface ablation refractive surgery:A six-month follow-up. Exp Eye Res. 2020;193:107976. doi: 10.1016/j.exer.2020.107976. doi: 10.1016/j.exer.2020.107976. [DOI] [PubMed] [Google Scholar]
- 63.Li B, Tian Y, Wang S. The correlation of cytokines and sensory hypersensitivity in mild dry eye patients characterized by symptoms outweighing signs. Mol Vis. 2020;26:359–9. [PMC free article] [PubMed] [Google Scholar]
- 64.Li Q, Liu J, Liu C, Piao J, Yang W, An N, et al. Effects of intense pulsed light treatment on tear cytokines and clinical outcomes in meibomian gland dysfunction. PLoS One. 2021;16:e0256533. doi: 10.1371/journal.pone.0256533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X, Yin Y, Yue L, Gong L. Selective serotonin reuptake inhibitors aggravate depression-associated dry eye via activating the NF-kappaB pathway. Invest Ophthalmol Vis Sci. 2019;60:407–19. doi: 10.1167/iovs.18-25572. [DOI] [PubMed] [Google Scholar]
- 66.Jee D, Park SH, Kim MS, Kim EC. Antioxidant and inflammatory cytokine in tears of patients with dry eye syndrome treated with preservative-free versus preserved eye drops. Invest Ophthalmol Vis Sci. 2014;55:5081–9. doi: 10.1167/iovs.14-14483. [DOI] [PubMed] [Google Scholar]
- 67.Lu R, Huang R, Li K, Zhang X, Yang H, Quan Y, et al. The influence of benign essential blepharospasm on dry eye disease and ocular inflammation. Am J Ophthalmol. 2014;157:591–7.e591-2. doi: 10.1016/j.ajo.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 68.Tan X, Sun S, Liu Y, Zhu T, Wang K, Ren T, et al. Analysis of Th17-associated cytokines in tears of patients with dry eye syndrome. Eye (Lond) 2014;28:608–13. doi: 10.1038/eye.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mrugacz M, Ostrowska L, Bryl A, Szulc A, Zelazowska-Rutkowska B, Mrugacz G. Pro-inflammatory cytokines associated with clinical severity of dry eye disease of patients with depression. Adv Med Sci. 2017;62:338–44. doi: 10.1016/j.advms.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 70.Schargus M, Langhorst CA, Joachim S, Frings A, Krause K, Reifenberger J, et al. Hidradenitis suppurativa is associated with symptoms of keratoconjunctivitis sicca. Curr Eye Res. 2021;46:23–30. doi: 10.1080/02713683.2020.1775259. [DOI] [PubMed] [Google Scholar]
- 71.Liu R, Ma B, Gao Y, Ma B, Liu Y, Qi H. Tear inflammatory cytokines analysis and clinical correlations in diabetes and nondiabetes with dry eye. Am J Ophthalmol. 2019;200:10–5. doi: 10.1016/j.ajo.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Yang T, Ma B, Xie J, Zhou Y, Liu R, Duan H, et al. Evaluation of ocular surface characteristics in dry eye disease with and without soft contact lens wear:A comparative study. Eye Contact Lens. 2022;48:377–83. doi: 10.1097/ICL.0000000000000904. [DOI] [PubMed] [Google Scholar]
- 73.Ren Y, Feng J, Lin Y, Reinach PS, Liu Y, Xia X, et al. MiR-223 inhibits hyperosmolarity-induced inflammation through down regulating NLRP3 activation in human corneal epithelial cells and dry eye patients. Exp Eye Res. 2022;220:109096. doi: 10.1016/j.exer.2022.109096. doi: 10.1016/j.exer.2022.109096. [DOI] [PubMed] [Google Scholar]
- 74.Niu L, Zhang S, Wu J, Chen L, Wang Y. Upregulation of NLRP3 inflammasome in the tears and ocular surface of dry eye patients. PLoS One. 2015;10:e0126277. doi: 10.1371/journal.pone.0126277. doi:10.1371/journal.pone.0126277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.D'Souza S, Padmanabhan Nair A, Iyappan G, Dickman MM, Thakur P, Mullick R, et al. Clinical and molecular outcomes after combined intense pulsed light therapy with low-level light therapy in recalcitrant evaporative dry eye disease with meibomian gland dysfunction. Cornea. 2022;41:1080–7. doi: 10.1097/ICO.0000000000002954. [DOI] [PubMed] [Google Scholar]
- 76.Jee D, Park M, Lee HJ, Kim MS, Kim EC. Comparison of treatment with preservative-free versus preserved sodium hyaluronate 0.1% and fluorometholone 0.1% eye drops after cataract surgery in patients with preexisting dry-eye syndrome. J Cataract Refract Surg. 2015;41:756–63. doi: 10.1016/j.jcrs.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 77.Li Z, Wang H, Liang M, Li Z, Li Y, Zhou X, et al. Hypochlorous acid can be the novel option for the meibomian gland dysfunction dry eye through ultrasonic atomization. Dis Markers. 2022;2022:8631038. doi: 10.1155/2022/8631038. doi:10.1155/2022/8631038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dionne K, Redfern RL, Nichols JJ, Nichols KK. Analysis of tear inflammatory mediators:A comparison between the microarray and Luminex methods. Mol Vis. 2016;22:177–88. [PMC free article] [PubMed] [Google Scholar]
- 79.Moon SY, Han SA, Kwon HJ, Park SY, Lee JH, Chung HS, et al. Effects of lid debris debridement combined with meibomian gland expression on the ocular surface MMP-9 levels and clinical outcomes in moderate and severe meibomian gland dysfunction. BMC Ophthalmol. 2021;21:175. doi: 10.1186/s12886-021-01926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu Z, Liu T, Zhou X, Yang Y, Liu Y, Zhou H, et al. Rapid and quantitative detection of tear MMP-9 for dry eye patients using a novel silicon nanowire-based biosensor. Biosens Bioelectron. 2022;214:114498. doi: 10.1016/j.bios.2022.114498. doi:10.1016/j.bios.2022.114498. [DOI] [PubMed] [Google Scholar]
- 81.Choi MG, Yeo JH, Kang JW, Chun YS, Lee JK, Kim JC. Effects of botulinum toxin type A on the treatment of dry eye disease and tear cytokines. Graefes Arch Clin Exp Ophthalmol. 2019;257:331–8. doi: 10.1007/s00417-018-4194-3. [DOI] [PubMed] [Google Scholar]
- 82.Uchino Y, Mauris J, Woodward AM, Dieckow J, Amparo F, Dana R, et al. Alteration of galectin-3 in tears of patients with dry eye disease. Am J Ophthalmol. 2015;159:1027–35.e1023. doi: 10.1016/j.ajo.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Messmer EM, von Lindenfels V, Garbe A, Kampik A. Matrix metalloproteinase 9 testing in dry eye disease using a commercially available point-of-care immunoassay. Ophthalmology. 2016;123:2300–8. doi: 10.1016/j.ophtha.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 84.Tong L, Beuerman R, Simonyi S, Hollander DA, Stern ME. Effects of punctal occlusion on clinical signs and symptoms and on tear cytokine levels in patients with dry eye. Ocul Surf. 2016;14:233–41. doi: 10.1016/j.jtos.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 85.Chan TC, Ye C, Chan KP, Chu KO, Jhanji V. Evaluation of point-of-care test for elevated tear matrix metalloproteinase 9 in post-LASIK dry eyes. Br J Ophthalmol. 2016;100:1188–91. doi: 10.1136/bjophthalmol-2015-307607. [DOI] [PubMed] [Google Scholar]
- 86.Soria J, Acera A, Merayo LJ, Duran JA, Gonzalez N, Rodriguez S, et al. Tear proteome analysis in ocular surface diseases using label-free LC-MS/MS and multiplexed-microarray biomarker validation. Sci Rep. 2017;7:17478. doi: 10.1038/s41598-017-17536-2. doi:10.1038/s41598-017-17536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kook KY, Jin R, Li L, Yoon HJ, Yoon KC. Tear osmolarity and matrix metallopeptidase-9 in dry eye associated with Sjogren's syndrome. Korean J Ophthalmol. 2020;34:179–86. doi: 10.3341/kjo.2019.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tong AY, Passi SF, Gupta PK. Clinical outcomes of lifitegrast 5% ophthalmic solution in the treatment of dry eye disease. Eye Contact Lens. 2020;46((Suppl 1)):S20–4. doi: 10.1097/ICL.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 89.Mullick R, Annavajjhala S, Thakur P, Mohapatra A, Shetty R, D'Souza S. Efficacy of topical cyclosporine 0.05% and osmoprotective lubricating eye drops in treating dry eye disease and inflammation. Indian J Ophthalmol. 2021;69:3473–7. doi: 10.4103/ijo.IJO_3822_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee YH, Bang SP, Shim KY, Son MJ, Kim H, Jun JH. Association of tear matrix metalloproteinase 9 immunoassay with signs and symptoms of dry eye disease:A cross-sectional study using qualitative, semiquantitative, and quantitative strategies. PLoS One. 2021;16:e0258203. doi: 10.1371/journal.pone.0258203. doi:10.1371/journal.pone.0258203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alven A, Lema C, Redfern RL. Impact of low humidity on damage-associated molecular patterns at the ocular surface during dry eye disease. Optom Vis Sci. 2021;98:1231–8. doi: 10.1097/OPX.0000000000001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alghamdi W, Markoulli M, Papas E. The relationship between tear film MMP-9 and meibomian gland changes during soft contact lens wear. Cont Lens Anterior Eye. 2020;43:154–8. doi: 10.1016/j.clae.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 93.Masli S, Akpek EK. Reduced tear thrombospondin-1/matrix metalloproteinase-9 ratio can aid in detecting Sjogren's syndrome etiology in patients with dry eye. Clin Transl Sci. 2022;15:1999–2009. doi: 10.1111/cts.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Minarikova M, Fik Z, Storm J, Helisova K, Ferrova K, Mahelkova G. Tear matrix metalloproteinase-9 levels may help to follow a ocular surface injury in lagophthalmic eyes. PLoS One. 2022;17:e0274173. doi: 10.1371/journal.pone.0274173. doi:10.1371/journal.pone.0274173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soifer M, Mousa HM, Stinnett SS, Galor A, Perez VL. Matrix metalloproteinase 9 positivity predicts long term decreased tear production. Ocul Surf. 2021;19:270–4. doi: 10.1016/j.jtos.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 96.Peart DJ, Walshe IH, Sweeney EL, James E, Henderson T, O'Doherty AF, et al. The effect of acute exercise on environmentally induced symptoms of dry eye. Physiol Rep. 2020;8:e14262. doi: 10.14814/phy2.14262. doi:10.14814/phy2.14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Postnikoff CK, Held K, Viswanath V, Nichols KK. Enhanced closed eye neutrophil degranulation in dry eye disease. Ocul Surf. 2020;18:841–51. doi: 10.1016/j.jtos.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 98.Kuo MT, Fang PC, Chao TL, Chen A, Lai YH, Huang YT, et al. Tear proteomics approach to monitoring Sjogren syndrome or dry eye disease. Int J Mol Sci. 2019;20:1932. doi: 10.3390/ijms20081932. doi:10.3390/ijms20081932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Acera A, Vecino E, Duran JA. Tear MMP-9 levels as a marker of ocular surface inflammation in conjunctivochalasis. Invest Ophthalmol Vis Sci. 2013;54:8285–91. doi: 10.1167/iovs.13-12235. [DOI] [PubMed] [Google Scholar]
- 100.Epitropoulos AT, Donnenfeld ED, Shah ZA, Holland EJ, Gross M, Faulkner WJ, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. 2016;35:1185–91. doi: 10.1097/ICO.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schargus M, Ivanova S, Stute G, Dick HB, Joachim SC. Comparable effects on tear film parameters after femtosecond laser-assisted and conventional cataract surgery. Int Ophthalmol. 2020;40:3097–104. doi: 10.1007/s10792-020-01532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chao WW, Tan SQ, Liu JH, Chen MM, Shiu HW, Chao HM. Dry eye:The effect of Chi-Ju-Di-Huang-Wan Plus Si Wu Tang and the underlying mechanism. J Altern Complement Med. 2020;26:138–46. doi: 10.1089/acm.2019.0201. [DOI] [PubMed] [Google Scholar]
- 103.Pflugfelder SC, Stern M, Zhang S, Shojaei A. LFA-1/ICAM-1 interaction as a therapeutic target in dry eye disease. J Ocul Pharmacol Ther. 2017;33:5–12. doi: 10.1089/jop.2016.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Black RA. Tumor necrosis factor-alpha converting enzyme. Int J Biochem Cell Biol. 2002;34:1–5. doi: 10.1016/s1357-2725(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 105.Sancho Ferrando E, Hanslin K, Hultstrom M, Larsson A, Frithiof R, Lipcsey M, et al. Soluble TNF receptors predict acute kidney injury and mortality in critically ill COVID-19 patients:A prospective observational study. Cytokine. 2022;149:155727. doi: 10.1016/j.cyto.2021.155727. doi: 10.1016/j.cyto. 2021.155727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Geering B, Gurzeler U, Federzoni E, Kaufmann T, Simon HU. A novel TNFR1-triggered apoptosis pathway mediated by class IA PI3Ks in neutrophils. Blood. 2011;117:5953–62. doi: 10.1182/blood-2010-11-322206. [DOI] [PubMed] [Google Scholar]
- 107.He M, Lippestad M, Li D, Hodges RR, Utheim TP, Dartt DA. Activation of the EGF receptor by histamine receptor subtypes stimulates mucin secretion in conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2018;59:3543–53. doi: 10.1167/iovs.18-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Portal C, Gouyer V, Gottrand F, Desseyn JL. Ocular mucins in dry eye disease. Exp Eye Res. 2019;186:107724. doi: 10.1016/j.exer.2019.107724. doi: 10.1016/j.exer. 2019.107724. [DOI] [PubMed] [Google Scholar]
- 109.Narimatsu A, Hattori T, Koike N, Tajima K, Nakagawa H, Yamakawa N, et al. Corneal lymphangiogenesis ameliorates corneal inflammation and edema in late stage of bacterial keratitis. Sci Rep. 2019;9:2984. doi: 10.1038/s41598-019-39876-x. doi:10.1038/s41598-019-39876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dohlman TH, Ding J, Dana R, Chauhan SK. T cell-derived granulocyte-macrophage colony-stimulating factor contributes to dry eye disease pathogenesis by promoting CD11b+myeloid cell maturation and migration. Invest Ophthalmol Vis Sci. 2017;58:1330–6. doi: 10.1167/iovs.16-20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoon HJ, Jang WH, An S, Ji YS, Yoon KC. Tear neuromediators in subjects with and without dry eye according to ocular sensitivity. Chonnam Med J. 2022;58:37–42. doi: 10.4068/cmj.2022.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Urbak L, Vorum H. Heat shock proteins in the human eye. Int J Proteomics. 2010;2010:479571. doi: 10.1155/2010/479571. doi:10.1155/2010/479571. [DOI] [PMC free article] [PubMed] [Google Scholar]