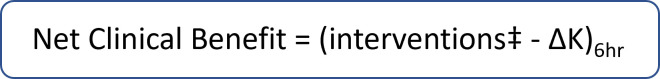Abstract
Introduction
Hyperkalaemia is common, life-threatening and often requires emergency department (ED) management; however, no standardised ED treatment protocol exists. Common treatments transiently reducing serum potassium (K+) (including albuterol, glucose and insulin) may cause hypoglycaemia. We outline the design and rationale of the Patiromer Utility as an Adjunct Treatment in Patients Needing Urgent Hyperkalaemia Management (PLATINUM) study, which will be the largest ED randomised controlled hyperkalaemia trial ever performed, enabling assessment of a standardised approach to hyperkalaemia management, as well as establishing a new evaluation parameter (net clinical benefit) for acute hyperkalaemia treatment investigations.
Methods and analysis
PLATINUM is a Phase 4, multicentre, randomised, double-blind, placebo-controlled study in participants who present to the ED at approximately 30 US sites. Approximately 300 adult participants with hyperkalaemia (K+ ≥5.8 mEq/L) will be enrolled. Participants will be randomised 1:1 to receive glucose (25 g intravenously <15 min before insulin), insulin (5 units intravenous bolus) and aerosolised albuterol (10 mg over 30 min), followed by a single oral dose of either 25.2 g patiromer or placebo, with a second dose of patiromer (8.4 g) or placebo after 24 hours. The primary endpoint is net clinical benefit, defined as the mean change in the number of additional interventions less the mean change in serum K+, at hour 6. Secondary endpoints are net clinical benefit at hour 4, proportion of participants without additional K+-related medical interventions, number of additional K+-related interventions and proportion of participants with sustained K+ reduction (K+ ≤5.5 mEq/L). Safety endpoints are the incidence of adverse events, and severity of changes in serum K+ and magnesium.
Ethics and dissemination
A central Institutional Review Board (IRB) and Ethics Committee provided protocol approval (#20201569), with subsequent approval by local IRBs at each site, and participants will provide written consent. Primary results will be published in peer-reviewed manuscripts promptly following study completion.
Trial registration number
Keywords: hypertension, clinical trials, heart failure, accident & emergency medicine
Strengths and limitations of this study.
PLATINUM is planned to be the largest emergency department (ED) randomised controlled hyperkalaemia trial ever performed.
This study will provide the opportunity to assess a standardised approach to hyperkalaemia management.
The study will also establish a new evaluation parameter (ie, net clinical benefit) for acute hyperkalaemia treatment investigations.
Limitations include the difficulties of patient recruitment in an ED environment, and that further studies may be required to assess the benefit of patiromer as an adjunct treatment to other ED hyperkalaemia therapies (other than the protocol-specified standard of care).
Introduction
Hyperkalaemia, generally defined as serum potassium (K+) >5.5 mEq/L, is common, can lead to life-threatening cardiac arrhythmias and frequently affects patients in the emergency department (ED).1–3 In 2014, more than 1 million ED visits had an International Classification of Diseases (ninth edition) code related to hyperkalaemia,4 with emergent hyperkalaemia likely to rise in parallel with increasing prevalence of hyperkalaemia risk factors5 (eg, chronic kidney disease,6 7 heart failure8 and hypertension9). In addition, many patients have recurrent hyperkalaemia following discharge from the ED.10 Expert panel recommendations and treatment algorithms for the management of hyperkalaemia11–14 exist; however, there is no standardised US protocol for ED hyperkalaemia management.15 Common medications currently used to treat hyperkalaemia in the ED, such as nebulised albuterol and intravenous insulin, with or without glucose,11 12 14–24 often cause adverse events (AEs), such as hypoglycaemia or hyperglycaemia.15–19 25 Additionally, treatments that only shift K+ into the cell, rather than remove it, frequently result in recurrence of hyperkalaemia 2–3 hours after treatment,24 26 particularly in patients undergoing haemodialysis.7 Repeat treatment to counter hyperkalaemia recurrence then further increases the risk of AEs.15–19 25
Alternatively, the use of K+ binders to eliminate K+ may be a better treatment strategy for emergent hyperkalaemia, although the current evidence lacks evaluation in a large randomised controlled trial.24 Two small, randomised studies (REDUCE and ENERGIZE) have shown promising results by adding either patiromer or sodium zirconium cyclosilicate to insulin and glucose therapy or investigator-designated standard of care (SOC); however, these studies were statistically inconclusive.27 28 Sodium polystyrene sulfonate (SPS) is a historically established treatment for chronic hyperkalaemia, reducing serum K+ via colonic excretion.29 However, the onset of action, degree of K+ lowering and patient tolerance of SPS are unpredictable.7 30 31 Loop diuretics are commonly used in management of acute hyperkalaemia; however, there is a lack of clinical studies to support their use in this setting.2 Ultimately, dialysis represents a definitive treatment for hyperkalaemia; however, effective management of hyperkalaemia through dialysis is complex and challenging.7 Thus, the new oral K+ binders with fewer adverse effects, such as patiromer, may offer a solution for the removal of excess K+ in hyperkalaemic patients presenting to the ED. Patiromer is a non-absorbed, oral K+ binder using sodium-free exchange32 with efficacy in the treatment of hyperkalaemia in patients with chronic kidney disease and heart failure21 28 33–37 and approval for use in the USA22 and European Union23 for treatment of hyperkalaemia. Given the variability of hyperkalaemia treatment in the ED,15 the challenge of emergent dialysis,7 and the serious risks of AEs with insulin treatment,15–19 25 there is a need for evaluation of novel K+ binders as additional treatments in the ED that act to remove excess K+,38 39 which have fewer AEs.
The PLATINUM trial will employ a systematic approach to investigate the use of patiromer as an adjunct treatment in hyperkalaemic patients presenting to the ED. The primary objective is to determine if patiromer, as adjunct to intravenous insulin, glucose and inhaled beta-agonist therapy, lowers K+ and reduces the need for additional medical interventions for the management of hyperkalaemia. Secondary objectives are to determine if adjunctive treatment with patiromer results in fewer additional K+-related medical interventions, enables a sustained reduction in K+ without additional medical interventions, and leads to a sustained reduction in K+ 24 hours after ED discharge.
Methods and analysis
Study design
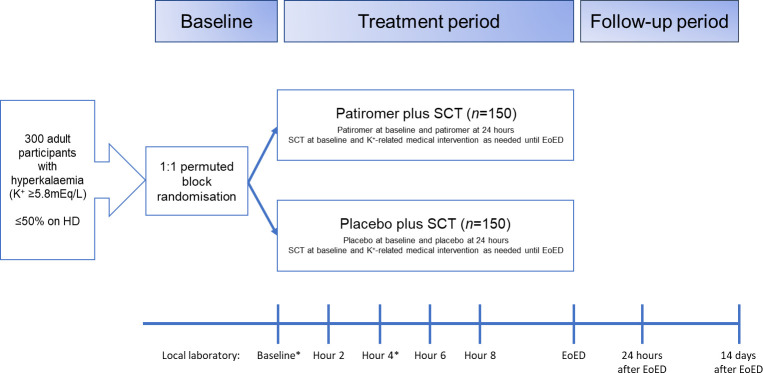
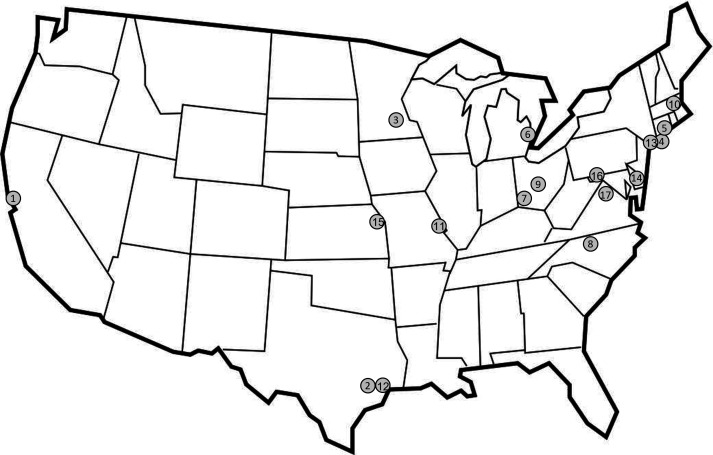
This is a Phase 4, multicentre, randomised, double-blind, placebo-controlled, parallel-group study (figure 1). It is planned that PLATINUM will enrol approximately 300 participants with hyperkalaemia at about 30 ED sites in the USA (figure 2). The schedule of assessments is shown in table 1.
Figure 1.
PLATINUM study design. *Local laboratory or point-of-care testing may be used to confirm eligibility for the study. K+ testing can be repeated at the discretion of the investigator at any time during the treatment period but is required at hour 4. ED, emergency department; EoED, end of emergency department stay (defined as discharge from the ED or initiation of dialysis); HD, haemodialysis; SCT, standard combination therapy (defined as 5 U intravenous insulin, 25 g intravenous glucose and 10 mg aerosolised albuterol) and K+-related medical intervention (defined as additional administration of insulin, glucose or albuterol (or their combination) at any dose, or any other K+-lowering medication can be initiated and repeated at the discretion of the investigator at any time); however, SCT is recommended.
Figure 2.
Proposed study centre locations. Study centres actively enrolling participants are shown on map. (1) Stanford University School of Medicine, California; (2) Henry JN Taub Hospital/Baylor College of Medicine, Texas; (3) Hennepin County Medical Center, University of Minnesota, Minnesota; (4) Stony Brook University Hospital, Stony Brook, New York; (5) Yale University, Connecticut; (6) Henry Ford Hospital, Michigan; (7) University of Cincinnati, Ohio; (8) Wake Forest University, North Carolina; (9) The Ohio State University Wexner Medical Center, Ohio; (10) Baystate Health, Massachusetts; (11) Washington University in St. Louis, Missouri; (12) UT Memorial Hermann Hospital, Texas Medical Center, Texas; (13) Mount Sinai, Icahn School of Medicine, New York; (14) Cristiana Care, Wilmington, Delaware; (15) University of Kansas Medical Center, Kansas; (16) Meritus Medical Center, Maryland; (17) George Washington University, Washington DC.
Table 1.
Assessment schedule during treatment period and follow-up
| Procedures | Baseline | Treatment period | Assessment 6/ET | Follow-up | ||||
| Assessment 1 | Assessment 2 | Assessment 3 | Assessment 4 | Assessment 5 | Assessment 7 | Assessment 8 | ||
| Hour 0* | Hour 2±15 min | Hour 4±15 min | Hour 6±15 min | Hour 8±15 min | Hour 10 or EoED† | Hour 0+30–48 hours | EoED +14 days +3 days |
|
| Informed consent | X | |||||||
| Eligibility criteria‡ | X | |||||||
| IWRS entry | X | |||||||
| Demographics | X | |||||||
| Medical/surgical history | X | |||||||
| Weight, height | X | |||||||
| Vital signs§ | X | |||||||
| ECG | X | X | ||||||
| Potassium level¶ | X | X | X | X | X | X | X | |
| Magnesium level | X | X | X | |||||
| Pregnancy test (for female participants) | X | |||||||
| Adverse events | X | X | X | X | X | X | X | X |
| Prior medications** | X | |||||||
| Concomitant medications | X | X | X | X | X | X | X | X |
| Randomisation | X | |||||||
| Administer study drug | X | X†† | ||||||
| Administer SCT‡‡ | X | Potassium-related medical intervention as needed, but prefer SCT | ||||||
*Hour 0 is defined as the time of study drug administration (study drug needs to be administered within 60 min of verifying eligible serum potassium and administering SCT).
†EoED is defined as discharge from the emergency department or initiation of dialysis, whichever occurs sooner.
‡Includes verbal check of pregnancy status for female participants. Pregnancy status to be confirmed for female participants via laboratory (blood or urine samples acceptable) at Assessment 1.
§Blood pressure, heart rate, pulse oximetry, respiratory rate and temperature.
¶Obtained from the laboratory only, not point of care.
**Up to 72 hours prior to baseline visit.
††A study drug packet will be given to participants to prepare and take 24 hours after the first dose is administered.
‡‡SCT is defined as insulin (5 U administered as a bolus), glucose (25 g administered intravenously <15 min before the insulin) and aerosolised albuterol (10 mg over 30 min) at baseline. Further potassium-related medical interventions, defined as additional administrations of insulin, glucose or albuterol (or their combination) at any dose, or any other potassium-lowering medication can be initiated and repeated at the discretion of the investigator at any time; however, SCT is preferred.
EoED, end of emergency department; ET, early termination; IWRS, Interactive Web Response System; SCT, standard combination therapy.
Impact of COVID-19
To minimise the impact of staffing and institution challenges resulting from the COVID-19 pandemic on enrolment, the trial has been extended by more than 2 years. Additional efforts to maintain enrolment include: new and total enrolment counts being sent to each site on a weekly basis; increased communication with primary investigators at each site, as well as regular primary investigator and research staff teleconferencing; and increased reimbursement to cover unanticipated costs associated with the pandemic.
Participants who are admitted to the ED with hyperkalaemia, provide informed consent and satisfy eligibility criteria, will be enrolled and undergo assessment.
The treatment period will be from the completion of the baseline assessment until discharge from the ED or initiation of dialysis, whichever occurs first. The expected duration of subject participation is 15 days; the treatment period is up to 1 day, and the follow-up period is 14 days. Participants who prematurely discontinue study drug will remain in the study to be monitored and assessed for safety and efficacy. The 14-day follow-up will be conducted via a phone call. Additional K+-related medical interventions, defined as post-baseline administration of insulin/glucose, with or without albuterol, or any other K+-lowering medication, can be initiated and repeated at any time during the treatment period at the discretion of the investigator or treating team. However, a standard combination therapy (SCT) is encouraged if additional K+-related interventions are needed.
The investigational drug was Food and Drug Administration (FDA) approved before any enrolments took place. However, insurance was obtained and maintained by the grantor and the sponsor to ensure the consequences of any unanticipated complications could be mitigated.
Participants
Eligible participants must be ≥18 years of age with hyperkalaemia, defined as K+ ≥5.8 mEq/L (chosen as the value where an intervention is required in the ED), obtained via local laboratory or point-of-care testing. Exclusion criteria include clinically significant arrhythmia, haemodynamic instability (defined as mean arterial pressure ≤65 mm Hg, or heart rate ≤40 or ≥125 beats per min), hyperkalaemia solely due to overdose of K+ supplements, known bowel obstruction, treatment with K+ binders in the 7 days prior to enrolment, expected dialysis during the first 6 hours of study treatment or enrolment, known hypersensitivity to patiromer or its ingredients, participation in any other investigational study <30 days prior to screening, life expectancy <6 months and pregnancy or breast feeding.
Study drug formulation
Patiromer sorbitex calcium (patiromer) or placebo (microcrystalline cellulose) will be stored between 2°C and 8°C and provided to the participant blinded, as a powder for oral suspension in packets.
Randomisation and treatment
Participants will be randomised 1:1 to 25.2 g of patiromer at baseline and 8.4 g 24 hours after the initial dose, or placebo, in addition to SCT, using permuted block randomisation, stratified by baseline chronic dialysis status (on dialysis vs not on dialysis). A maximum of 50% of participants will be on chronic dialysis. Randomisation will be by a centralised list accessed electronically via an interactive web response system at baseline. Immediately following baseline procedures and randomisation, participants will be administered SCT consisting of glucose (25 g intravenously <15 min before insulin) given if the blood sugar is below 400 mg/dL, insulin (5 units administered as an intravenous bolus) and aerosolised albuterol (10 mg over 30 min). Participants then receive a single oral dose of study drug (25.2 g) at baseline (patiromer or placebo). Participants, site personnel, clinical providers and the sponsor will be blinded to the study drug. The clinical trial supply management team will provide blinded sachets of patiromer and placebo, and the site investigational pharmacists will maintain the blinding. In the case of a medical emergency, the investigator may request that the blind be broken if it is considered important to the management of the medical emergency, or for study-specific suspected unexpected serious adverse reaction and aggregate safety reporting to health authorities. In such cases, the investigator will be unblinded via the Interactive Web Response System. The study drug will be prepared immediately prior to administration and given at least 3 hours before or after other orally administered medications, if possible, in the ED. Study drug will be mixed with water, apple juice or cranberry juice only. A second dose of the same study drug will be administered 24 hours after the initial dose.
Endpoints
The primary endpoint is the net clinical benefit, as previously described in a post hoc analysis of the REDUCE study,40 defined as the mean change in the number of interventions less the change in serum K+, at hour 6 between the groups (figure 3). Interventions consist of additional K+-related medical interventions, defined as post-baseline administration of insulin, glucose or albuterol (or their combination) at any dose, or any other K+-lowering medication provided to participants at any time during the treatment period at the discretion of the investigator. Assessment of the efficacy of K+ binders in the ED can be confounded owing to repeat administrations of insulin and/or albuterol. Therefore, net clinical benefit is used to simultaneously assess both the number of additional K+-lowering medications required and the change in serum K+. Secondary endpoints are the net clinical benefit at hour 4; the proportion of participants without post-baseline K+-related medical interventions at hours 4, 6 and 8; the number of post-baseline K+-related medical interventions up until hours 6 and 8, and ED discharge; the proportion of participants with sustained K+ reduction (defined as K+ ≤5.5 mEq/L and 4 hours without K+-related medical intervention) at hours 6 and 8; and serum K+ 24 hours after ED discharge. An exploratory endpoint is the time to ED discharge. Safety endpoints are the incidence and severity of AEs, and changes from baseline in serum K+, magnesium and ECG. AEs and concomitant medications will be assessed every 2 hours after enrolment, until hour 10 or discharge from the ED; glucose checks are performed when clinically indicated by the medical team (a glucose check is not required by the protocol), for example, when a basic metabolic panel is drawn for K+ value, a glucose value will also be recorded.
Figure 3.
Primary endpoint: net clinical benefit. ‡Number of additional potassium (K+)-lowering interventions after initial treatment. ∆K will be determined from laboratory potassium (K+) values.
Statistical analysis
Based on the pilot study,28 a power calculation determined that a sample size of 60 participants per treatment arm provides 90% power to detect a difference in net clinical benefit at 6 hours (primary outcome) between the placebo and patiromer groups at two-sided α=0.05. Accounting for a potential treatment discontinuation rate of 60% by 6 hours, based on the nature of the disease and the need for emergent interventions beyond this protocol, 150 participants per treatment arm will be enrolled to reach the required sample size of 60 participants per arm for the final analysis.
The full analysis set (FAS) will consist of all participants who receive at least one dose of randomised treatment and have at least two post-baseline assessments or a 4-hour post-baseline blood draw. The FAS will be used for the evaluation of efficacy. The per-protocol set will consist of all participants who, in addition to the FAS criteria, have no major protocol deviations. The safety set will consist of all randomised participants who received at least one dose of study drug. Participants in the safety set will be analysed based on the study drug they received.
Net clinical benefit at hour 6 will be compared between groups using a Student’s t-test. A modified intention-to-treat analysis will be used for the primary endpoint, with an imputation method applied for missing data: participants who have been on placebo or patiromer for at least 4 hours will have the last observation carried forward to the hour 6 analysis. Secondary endpoints involving proportions of participants and counts of interventions will be analysed using the Cochran-Mantel-Haenszel method. Continuous variables (K+ level at specified time points) will be analysed using analysis of covariance methods. Kaplan-Meier curves will be used to analyse the time to ED discharge. Safety variables will consist of all AEs, clinical laboratory test results (serum K+ and magnesium), clinically significant ECG findings and reasons for discontinuing study drug. Abnormal ECGs or other safety assessments will qualify as an AE if they meet any of the following criteria: (1) it is accompanied by clinical symptoms or leads to a diagnosis (in such case the symptom or diagnosis will be recorded as an AE); (2) it results in a change in study treatment (eg, dosage modification, treatment interruption or treatment discontinuation); (3) it results in a medical intervention, a change in concomitant therapy or referral for further testing outside the protocol; (4) it is a clinically significant abnormality, as judged by the investigator.
Data management
An independent Data and Safety Monitoring Board/Data Monitoring Committee will not be established due to the short duration of the study. The integrity and quality of subject data will be ensured by providing training and process instructions for the completion of the electronic case report forms (eCRFs), performing quality control checks, conducting ongoing clinical data review (including medical and safety reviews) and performing source data verification and data reconciliation. The sponsor may conduct site monitoring visits at regular intervals in accordance with FDA and International Council for Harmonisation guidelines. The investigator will permit monitors to review and inspect facilities, and all records relevant to this study. The investigator will arrange for the retention of all study documentation (such as eCRF files or printed forms, research files and master files) for the duration specified in their respective site contract or as specified by the applicable regulatory authority, whichever is longer.
Patient and public involvement
None.
Ethics and dissemination
This study will be conducted according to the principles of the World Medical Association’s Declaration of Helsinki,41 and the amended International Council for Harmonisation Good Clinical Practice guidelines.42 The informed consent form for the study complies with the Declaration of Helsinki, federal regulations and International Council for Harmonisation guidelines; and was approved by the appropriate Institutional Review Board (IRB), Ethics Committee (EC) or Independent Ethics Committee (IEC). A copy of the consent form is shown in the supplement section (online supplemental file 1). Participants will provide consent in writing to the investigator or an authorised associate prior to study entry. The protocol (V.1.0, 20 March 2020) was approved by a central IRB (#20201569) and subsequently by the local IRB at each site. Each applicable regulatory authority/IRB/EC/IEC will review and approve amendments prior to their implementation. Primary results will be published in peer-reviewed manuscripts promptly following study completion. All authors will meet the International Committee of Medical Journal Editors requirements for authorship. A communications agency may provide editing of that manuscript, as well as administrative support for journal submission.
bmjopen-2022-071311supp001.pdf (138.3KB, pdf)
Standard clinical trials information can be found on ClinicalTrials.gov. There are no plans to grant public access to the participant-level data set or statistical code.
Discussion
Although hyperkalaemia is common and potentially life-threatening, there is no standardised ED treatment protocol.1–3 15 The efficacy and safety of many hyperkalaemia treatments are not well established in the ED, resulting in a considerable variation in treatment, which is not only detrimental to patients but hampers the ability to perform comparative assessments of the benefit of novel therapies.
The PLATINUM study will assess the benefit of adding patiromer to an SCT regimen: glucose (25 g intravenously <15 min before insulin), insulin (5 U administered as an intravenous bolus) and aerosolised albuterol (10 mg over 30 min). As some SCT agents temporarily shift K+ into the cells, repeat administration is commonly required to prevent rebound in serum K+ levels, increasing the risk of AEs.15–19 25 43 44 In contrast, the patiromer removes K+ via binding in the gastrointestinal tract.38 Of note, the PLATINUM study will use 5 U of insulin, as this has similar efficacy to 10 U.43
Recently, a retrospective cohort study of 881 unique encounters from EDs, inpatient units and intensive care units, reported that a single dose of patiromer monotherapy was associated with a significant reduction from baseline in serum K+ in non-emergent hyperkalaemia.45 An open-label, pilot study in participants randomised to SOC (according to individual practice pattern or hospital protocol) versus 25.2 g of patiromer plus SOC demonstrated a reduction in serum K+ within 2 hours of with the addition of patiromer; however, reduction in K+ was not statistically significant at 6 hours, likely due to the small sample size and large variability in mean change in serum K+.28
In a post hoc analysis of the REDUCE study,40 net clinical benefit was used to evaluate the efficacy of patiromer plus SOC, compared with SOC alone. Net clinical benefit was defined as the mean change in the number of additional interventions, less the mean change in serum K+. This novel method of assessing the effect of K+ binders considers the overall benefit of both lowering serum K+ and simultaneously reducing the number of interventions required. Hence, net clinical benefit combines two potential merits of a novel agent and will also be useful in future trials as a method to investigate the effect of K+ binders to treat hyperkalaemia.
The secondary endpoint, serum K+ 24 hours after ED discharge, will provide insight on the value of giving a second dose of patiromer at discharge from the ED. Importantly, this study may support a standardised care algorithm with consistent dosing, reporting efficacy and safety data from a large, randomised, multicentre trial.
The protocol has several limitations. First, subjects with hyperkalaemia are invariably critically ill and the ED is a challenging environment for enrolment in interventional trials and so the attrition rate is expected to be high. Second, SOC in hyperkalaemia is not well defined and in the absence of guidelines it will be difficult to control the SOC treatment regimen. Lastly, a successful enrolment requires an eligible K+, signed consent and administration of both SOC treatment and investigational drug to occur within 60 min and that time window can be challenging.
The PLATINUM study started enrolment in October 2020 and is expected to end May 2023. It is the largest ED randomised controlled hyperkalaemia trial ever performed, with the opportunity to assess a standardised approach to hyperkalaemia management, as well as establish a new evaluation parameter, the net clinical benefit, for acute hyperkalaemia treatment investigations.
Supplementary Material
Acknowledgments
This work was executed by Comprehensive Research Associates (Texas, USA). Medical writing support was provided by AXON Communications, UK, and funded by CSL Vifor.
Footnotes
Twitter: @BischofMD
Contributors: ZR: Creating the protocol, interpretation of data, editing the manuscript. JB: Interpretation of data, editing the manuscript. CMQ: Interpretation of data, editing the manuscript. YD: Site investigator, interpretation of data, editing the manuscript. BS: Site investigator, acquisition and interpretation of data, editing the manuscript. JJB: Site investigator, data collection, interpretation of data, editing the manuscript. BED: Site investigator, data collection, interpretation of data, editing the manuscript. CAH: Contributed to protocol (study design), editing the manuscript. MRW: Contributed to protocol, interpretation of data, editing the manuscript. AJS: Contributed to protocol, interpretation of data, editing the manuscript. SB: Site investigator, data collection, interpretation of data, editing the manuscript. KMS-R: Creating the protocol, data collection and management, interpretation of data, editing the manuscript. WFP: Creating the protocol, obtaining co-funding, editing the manuscript. All authors will have access to the final trial data set. There are no contractual agreements limiting such access.
Funding: This work was funded by Vifor Fresenius Medical Care Renal Pharma, Glattbrugg, Switzerland. The study was conceived by the primary investigator (ZR) and coauthors. Funding sources did not influence the study design, implementation or interpretation or reporting of results.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographical or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: ZR: Advisory board memberships (Cardiorenal disease) for AstraZeneca and CSL Vifor. JB: Employee and shareholder of CSL Vifor. CMQ: Employee and shareholder of CSL Vifor. YD: No relevant conflicts of interest to disclose. BS: NHLBI, CDC, Comprehensive Research Associates (institutional grants). JJB: No relevant conflicts of interest to disclose. BED: No relevant conflicts of interest to disclose. CAH: Personal fees (consultant) from AstraZeneca, Bayer, Diamedica, FibroGen, Merck, NxStage, Pfizer, Relypsa, University of Oxford, Bristol Myers Squibb; Grants from the University of British Columbia and Bristol Myers Squibb and the National Institutes of Health (NIDDK and NHLBI), and author royalties from UpToDate. Stock ownership in Johnson & Johnson, Merck, and Pfizer. Employee of Hennepin Healthcare. MRW: Consulting fees: CSL Vifor and AstraZeneca. Honoraria: CSL Vifor. AJS: Research grants: Comprehensive Research Associates. Consulting fees: AstraZeneca. SB: No relevant conflicts of interest to disclose. KMS-R: No relevant conflicts of interest to disclose. WFP: Research Grants: Brainbox, Instrument Labs, Salix. Consultant: Abbott, Brainbox, Instrument Labs, Janssen, Osler, Roche, Siemens, CSL Vifor. Stock/Ownership Interests: AseptiScope Inc, Brainbox Inc, Braincheck Inc, Coagulo Inc, Comprehensive Research Associates LLC, Comprehensive Research Management Inc, Emergencies in Medicine LLC, Fast Inc, Forrest Devices, Ischemia DX LLC, Lucia Inc, Prevencio Inc, RCE Technologies, ROMTech, ScPharma, Trivirum Inc, Upstream Inc.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Lemoine L, Le Bastard Q, Batard E, et al. An evidence-based narrative review of the emergency department management of acute hyperkalemia. J Emerg Med 2021;60:599–606. 10.1016/j.jemermed.2020.11.028 [DOI] [PubMed] [Google Scholar]
- 2.Lindner G, Burdmann EA, Clase CM, et al. Acute hyperkalemia in the emergency department: a summary from a Kidney Disease: Improving Global Outcomes conference. Eur J Emerg Med 2020;27:329–37. 10.1097/MEJ.0000000000000691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer AJ, Thode HC, Peacock WF. A retrospective study of emergency department potassium disturbances: severity, treatment, and outcomes. Clin Exp Emerg Med 2017;4:73–9. 10.15441/ceem.16.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quality AfHRa . Healthcare cost and utilization project (Hcupnet). n.d. Available: https://datatools.ahrq.gov/hcupnet
- 5.Hunter RW, Bailey MA. Hyperkalemia: pathophysiology, risk factors and consequences. Nephrol Dial Transplant 2019;34:iii2–11. 10.1093/ndt/gfz206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol 2019;1165:3–15. 10.1007/978-981-13-8871-2_1 [DOI] [PubMed] [Google Scholar]
- 7.Bansal S, Pergola PE. Current management of hyperkalemia in patients on dialysis. Kidney Int Rep 2020;5:779–89. 10.1016/j.ekir.2020.02.1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savarese G, Becher PM, Lund LH, et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res 2023;118:3272–87. 10.1093/cvr/cvac013 [DOI] [PubMed] [Google Scholar]
- 9.Dorans KS, Mills KT, Liu Y, et al. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline. J Am Heart Assoc 2018;7:e008888. 10.1161/JAHA.118.008888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis J, Israni R, Betts KA, et al. Real-world management of hyperkalemia in the emergency department: an electronic medical record analysis. Adv Ther 2022;39:1033–44. 10.1007/s12325-021-02017-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafique Z, Weir MR, Onuigbo M, et al. Expert panel recommendations for the identification and management of hyperkalemia and role of patiromer in patients with chronic kidney disease and heart failure. J Manag Care Spec Pharm 2017;23:S10–9. 10.18553/jmcp.2017.23.4-a.s10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rafique Z, Chouihed T, Mebazaa A, et al. Current treatment and unmet needs of hyperkalaemia in the emergency department. Eur Heart J Suppl 2019;21(Suppl A):A12–9. 10.1093/eurheartj/suy029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rafique Z, Peacock F, Armstead T, et al. Hyperkalemia management in the emergency department: an expert panel consensus. J Am Coll Emerg Phys Open 2021;2:e12572. 10.1002/emp2.12572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossignol P, Legrand M, Kosiborod M, et al. Emergency management of severe hyperkalemia: guideline for best practice and opportunities for the future. Pharmacol Res 2016;113:585–91. 10.1016/j.phrs.2016.09.039 [DOI] [PubMed] [Google Scholar]
- 15.Peacock WF, Rafique Z, Clark CL, et al. Real world evidence for treatment of hyperkalemia in the emergency department (REVEAL-ED): A multicenter, prospective, observational study. J Emerg Med 2018;55:741–50. 10.1016/j.jemermed.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 16.Aljabri A, Perona S, Alshibani M, et al. Blood glucose reduction in patients treated with insulin and dextrose for hyperkalaemia. Emerg Med J 2020;37:31–5. 10.1136/emermed-2019-208744 [DOI] [PubMed] [Google Scholar]
- 17.Farina N, Anderson C. Impact of dextrose dose on hypoglycemia development following treatment of hyperkalemia. Ther Adv Drug Saf 2018;9:323–9. 10.1177/2042098618768725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 2012;40:3251–76. 10.1097/CCM.0b013e3182653269 [DOI] [PubMed] [Google Scholar]
- 19.Scott NL, Klein LR, Cales E, et al. Hypoglycemia as a complication of intravenous insulin to treat hyperkalemia in the emergency Department. Am J Emerg Med 2019;37:209–13. 10.1016/j.ajem.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 20.Boughton CK, Dixon D, Goble E, et al. Preventing hypoglycemia following treatment of hyperkalemia in hospitalized patients. J Hosp Med 2019;14:284–7. 10.12788/jhm.3145 [DOI] [PubMed] [Google Scholar]
- 21.Piña IL, Yuan J, Ackourey G, et al. Effect of patiromer on serum potassium in hyperkalemic patients with heart failure: pooled analysis of 3 randomized trials. Prog Cardiovasc Dis 2020;63:656–61. 10.1016/j.pcad.2020.09.007 [DOI] [PubMed] [Google Scholar]
- 22.United States Food and Drug Administration . VELTASSA - patiromer powder, for suspension. [Google Scholar]
- 23.European Medicines Agency . Veltassa powder for oral suspension. Summary of product characteristics. [Google Scholar]
- 24.Mahoney BA, Smith WAD, Lo DS, et al. Emergency interventions for hyperkalaemia. Cochrane Database Syst Rev 2005;2005:CD003235. 10.1002/14651858.CD003235.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moussavi K, Garcia J, Tellez-Corrales E, et al. Reduced alternative insulin dosing in hyperkalemia: a meta‐analysis of effects on hypoglycemia and potassium reduction. Pharmacotherapy 2021;41:598–607. 10.1002/phar.2596 [DOI] [PubMed] [Google Scholar]
- 26.Elliott MJ, Ronksley PE, Clase CM, et al. Management of patients with acute hyperkalemia. CMAJ 2010;182:1631–5. 10.1503/cmaj.100461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peacock WF, Rafique Z, Vishnevskiy K, et al. Emergency potassium normalization treatment including sodium zirconium cyclosilicate: A phase II, randomized, double-blind, placebo-controlled study (ENERGIZE). Acad Emerg Med 2020;27:475–86. 10.1111/acem.13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rafique Z, Liu M, Staggers KA, et al. Patiromer for treatment of hyperkalemia in the emergency department: A pilot study. Acad Emerg Med 2020;27:54–60. 10.1111/acem.13868 [DOI] [PubMed] [Google Scholar]
- 29.Sodium Polystyrene Sulfonate (Kayexalate) [prescribing information].
- 30.United States Food and Drug Administration . Sodium polystyrene sulfonate prescribing information. [Google Scholar]
- 31.Chaitman M, Dixit D, Bridgeman MB. Potassium-binding agents for the clinical management of hyperkalemia. P T 2016;41:43–50. [PMC free article] [PubMed] [Google Scholar]
- 32.Blair HA. Patiromer: a review in hyperkalaemia. Clin Drug Investig 2018;38:785–94. 10.1007/s40261-018-0675-8 [DOI] [PubMed] [Google Scholar]
- 33.Ali W, Bakris G. Evolution of patiromer use: a review. Curr Cardiol Rep 2020;22:94. 10.1007/s11886-020-01342-w [DOI] [PubMed] [Google Scholar]
- 34.Bakris GL, Pitt B, Weir MR, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA 2015;314:151–61. 10.1001/jama.2015.7446 [DOI] [PubMed] [Google Scholar]
- 35.Bushinsky DA, Williams GH, Pitt B, et al. Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney disease and hyperkalemia. Kidney Int 2015;88:1427–33. 10.1038/ki.2015.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015;372:211–21. 10.1056/NEJMoa1410853 [DOI] [PubMed] [Google Scholar]
- 37.Pitt B, Anker SD, Bushinsky DA, et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J 2011;32:820–8. 10.1093/eurheartj/ehq502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Harrison SD, Cope MJ, et al. Mechanism of action and pharmacology of patiromer, a nonabsorbed cross-linked polymer that lowers serum potassium concentration in patients with hyperkalemia. J Cardiovasc Pharmacol Ther 2016;21:456–65. 10.1177/1074248416629549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cañas AE, Troutt HR, Jiang L, et al. A randomized study to compare oral potassium binders in the treatment of acute hyperkalemia. BMC Nephrol 2023;24:89. 10.1186/s12882-023-03145-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rafique Z, Budden J, Boone S. SAEM 22 abstracts: Net clinical benefit of patiromer: a post hoc analysis of the REDUCE trial. Acad Emerg Med 2022;29:S8–429. [Google Scholar]
- 41.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 42.International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) . ICH harmonised guideline. Guideline for good clinical practice E6(R)2, 2017. [Google Scholar]
- 43.Pierce DA, Russell G, Pirkle JL. Incidence of hypoglycemia in patients with low eGFR treated with insulin and dextrose for hyperkalemia. Ann Pharmacother 2015;49:1322–6. 10.1177/1060028015607559 [DOI] [PubMed] [Google Scholar]
- 44.Humphrey TJL, James G, Wilkinson IB, et al. Clinical outcomes associated with the emergency treatment of hyperkalaemia with intravenous insulin-dextrose. Eur J Intern Med 2022;95:87–92. 10.1016/j.ejim.2021.09.018 [DOI] [PubMed] [Google Scholar]
- 45.Di Palo KE, Sinnett MJ, Goriacko P. Assessment of patiromer monotherapy for hyperkalemia in an acute care setting. JAMA Netw Open 2022;5:e2145236. 10.1001/jamanetworkopen.2021.45236 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-071311supp001.pdf (138.3KB, pdf)