Figure 1.
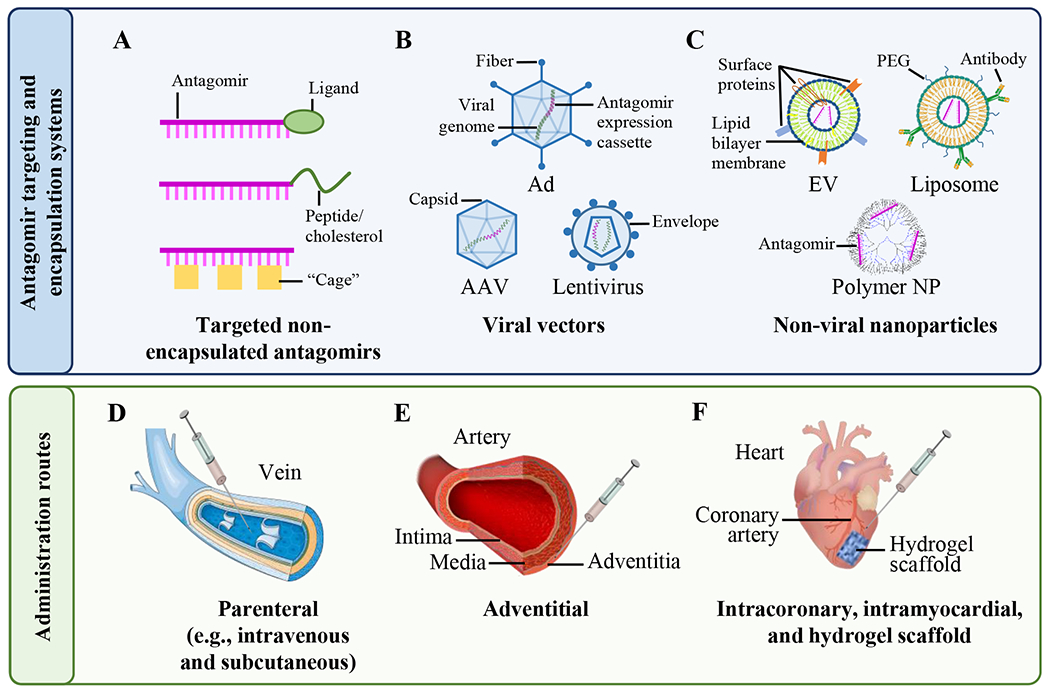
Schematic representation of targeted antagomir delivery systems and administration routes.
(Upper panel): Antagomirs (pink) are administered as non-encapsulated ASOs (A), are expressed from viral vectors (B), or are delivered by non-viral nanoparticle (NP) carriers (C). Some NP carriers can be loaded with plasmids (not shown) that express the antagomirs after cell entry. (A) Non-encapsulated antagomirs can be linked to functional groups such as ligands (e.g., N-acetylgalactosamine) that bind specific cell surface receptors or to cholesterol or cell-permeable peptides that enhance cell entry. Alternatively, photolabile “cages” can be linked to the antagomir nucleobases, allowing site-specific activation of the antagomir by illumination. (B) Antagomirs can also be expressed from viral vectors, typically adenoviruses (Ad), adeno-associated viruses (AAV), and lentiviruses (LV). In these cases, an expression cassette containing the antagomir sequence (pink) is inserted into the viral genome, potentially allowing long-term antagomir expression from within transduced cells. (C) Synthetic antagomirs (pink) or antagomir-encoding plasmids (not shown) can be incorporated within lipid-based NPs (e.g., extracellular vesicles (EVs), liposomes, or polymer-based NPs). Lower panel: Antagomirs are commonly administered via intravenous injection (D), subcutaneously (not shown), or by injection into the adventitia (E). Antagomirs can also be injected directly into the myocardium or applied to the epicardium using hydrogel scaffolds (F). Intracoronary administration (not shown) is also used to deliver antagomirs to the myocardium. This figure was partly generated using Servier Medical Art.
