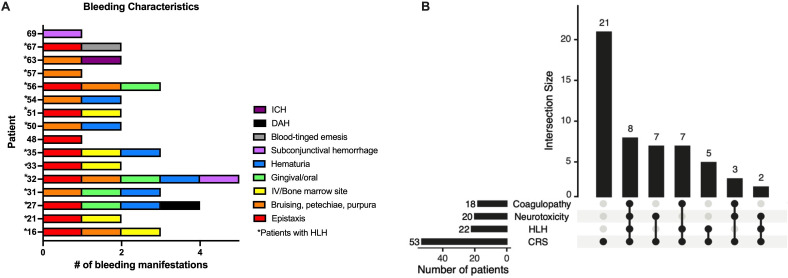
Figure 1.
Characteristics of patients with hematologic toxicities. (A) Bleeding manifestations of each patient who experienced clinically relevant bleeding after receiving CD22 CAR T-cell infusion. (*) Patients with carHLH. (B) Number of patients who experienced one clinically relevant toxicity of coagulopathy, carHLH or neurotoxicity and those that had more than one manifestation. Five of the 21 patients who did not experience coagulopathy, carHLH or neurotoxicity were unevaluable for coagulopathy, but presumed to not be coagulopathic. CAR, chimeric antigen receptor; carHLH, CAR-associated HLH-like toxicity; CRS, cytokine release syndrome; DAH, diffuse alveolar hemorrhage; HLH, hemophagocytic lymphohistiocytosis; ICH, intracranial hemorrhage; IV, intravenous line site.