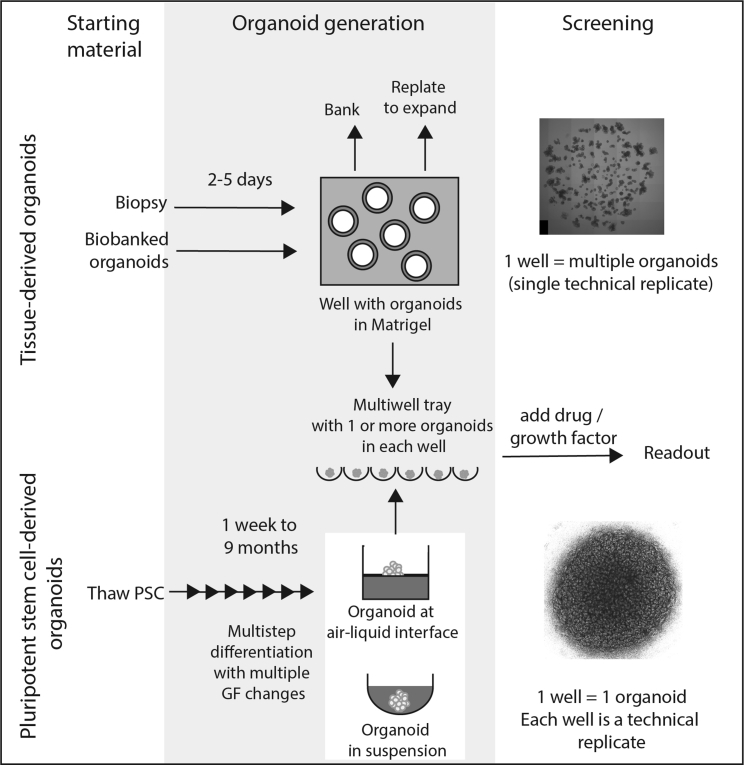
Figure 2.
Overview of organoid generation and experimental design for both tissue-derived and pluripotent stem cell-derived organoids
Tissue-derived organoid experiments can commence using fresh biopsy material but would normally involve an initial expansion and potentially a banking step in order to generate sufficient patient lines for experimental analysis. Features of this type of experiment include a short organoid generation time frame, capacity for extensive passaging, and ready freeze thawing of starting material. Organoids can be generated per well but are frequently cultured via plating into Matrigel with individual cells giving rise to an individual organoid and hence many organoids arising per well. Despite being smaller than PSC-derived organoids, these remain quite large multicellular structures with high-content imaging challenges for developing quantitative phenotypic readouts. PSC-derived organoid experiments all commence with pluripotent stem cells followed by extensive multi-step differentiation protocols, each of which are tailored for a specific tissue endpoint. These are usually larger structures, often with one organoid per well, microwell, or Transwell. Features of this type of experiment include the requirement for extended differentiation prior to experimental analysis. Their size and complexity can make image-based readouts challenging. There is a higher risk of off-target cell types and variability between organoids. Top image shows adult intestinal epithelial organoids derived from the ilium of a healthy individual cultured in a droplet of Matrigel. Bottom image shows a kidney organoid cultured on a Transwell filter.