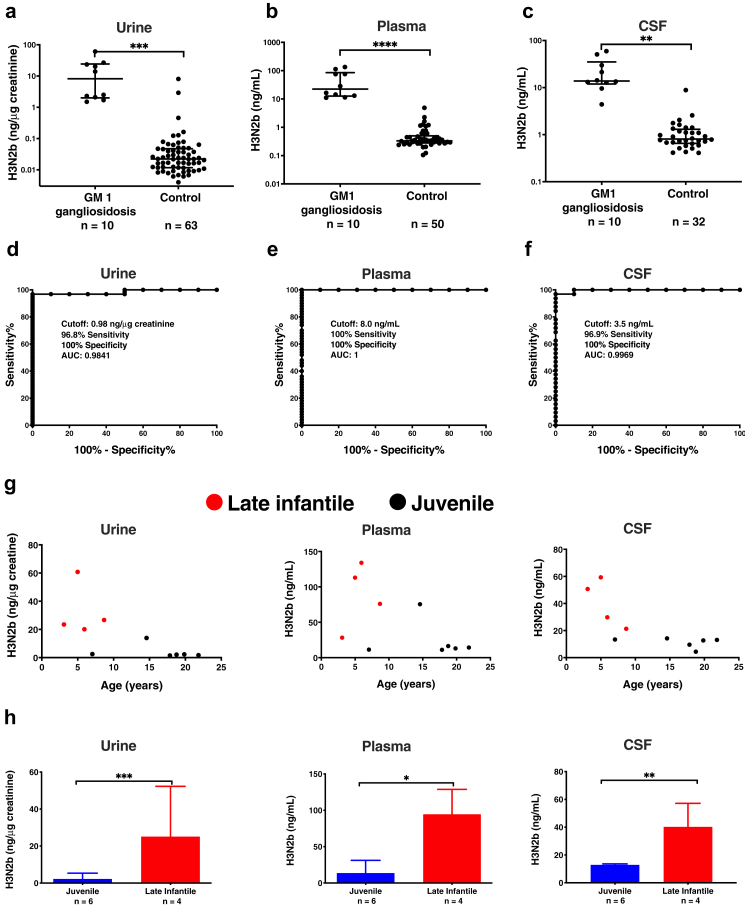
Fig. 5.
H3N2b in human urine, plasma, and CSF. (a) H3N2b normalized to creatinine in human urine samples. H3N2b concentrations are presented as median ± interquartile on log scale. Mann–Whitney U-test was used to compare GM1 gangliosidosis and controls. (b) H3N2b concentrations in human plasma samples. The measured H3N2b concentrations that were below the lower limit of quantification (LLOQ, 1 ng/mL) were used for purpose of plotting, though the CV and relative error (RE) for these samples may not meet acceptance criteria for the validated assay. H3N2b concentrations are presented as median ± interquartile on log scale. Mann–Whitney U-test was used to compare GM1 gangliosidosis and controls. (c) H3N2b concentrations in human CSF samples. The measured H3N2b concentrations that were below the LLOQ (1 ng/mL) were used for purpose of plotting, though the CV and RE for these samples may not meet acceptance criteria for the validated assay. H3N2b concentrations are presented as median ± interquartile on log scale. Mann–Whitney U-test was used to compare GM1 gangliosidosis and controls. (d) Determination of cutoff for urine using ROC curve. (e) Determination of cutoff for plasma using ROC curve. (f) Determination of cutoff for CSF using ROC curve. (g) Relations between urine, plasma, and CSF H3N2b and age/disease type. (h) Comparison of H3N2b in late infantile and juvenile GM1 gangliosidosis patients. Data are presented as mean ± standard deviation. Mann–Whitney U-test was used to compare late infantile and juvenile GM1 gangliosidosis patients. ∗: p < 0.05; ∗∗: p < 0.01; ∗∗∗: p < 0.001; ∗∗∗∗: p < 0.0001.