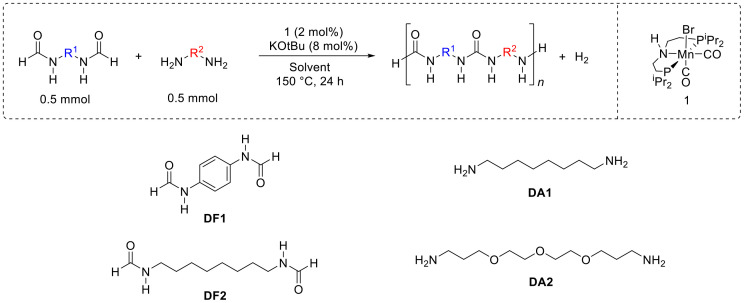
Catalytic conditions for the synthesis of polyureas from diformamides and diaminesa.
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Diformamide | Diamine | Base | Solvent | N2 | Yield/% | M n (MALDI) | Al : Ar | T d/°C | T m/°C |
| 1 | DF1 | DA1 | KOtBu | THF | Sealed | 82 | 1877 | 1.67 | 246 | 221 |
| 2 | DF1 | DA1 | KOtBu | Anisole | Sealed | 77 | 2111 | 1.03 | 246 | 220 |
| 3 | DF1 | DA1 | KOtBu | Anisole | Open | 32 | 2111 | 1.67 | 237 | 201 |
| 4 | DF1 | DA1 | KOtBu | Diglyme | Open | 79 | 1904 | 2.01 | 238 | 212 |
| 5 | DF1 | DA1 | KOtBu | DMSO | Open | 44 | — | 4.88 | 266 | 211 |
| 6 | DF1 | DA1 | K2CO3 | Anisole | Open | 30 | 1770 | 1.73 | 242 | 204 |
| 7 | DF2 | DA1 | KOtBu | Anisole | Open | 85 | 2111 | N/A | 253 | 216 |
| 8 | DF2 | DA1 | KOtBu | Diglyme | Open | 25 | 2110 | N/A | 213 | 210 |
| 9 | DF2 | DA1 | KOtBu | THF | Open | 78 | 2594 | N/A | 247 | 215 |
| 10 | DF1 | DA2 | KOtBu | Anisole | Open | 94 | 3140b | 1.16 | 223 | — |
Catalytic conditions: diamine (0.5 mmol), diformamide (0.5 mmol), solvent (2 mL), complex 1 (2 mol%), and KOtBu (8 mol%). Al : Ar = aliphatic : aromatic NMR integral relationship. Mn (MALDI, g mol−1): value is estimated as the maximum signal observed in MALDI FT-ICR mass spectrometry. Td (decomposition temperature) was recorded at 5% mass loss. Tm stands for melting temperature.
M n of 44 000 g mol−1 was estimated using GPC.
