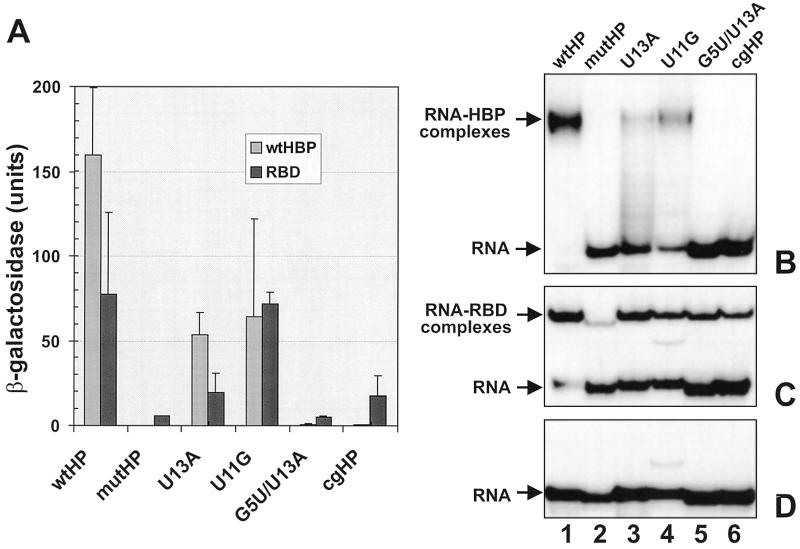
Figure 3.
Binding of full-length human HBP (wtHBP) and its minimal RBD to different hairpin structures. (A) Activation of the lacZ gene in the yeast three-hybrid system. Either wtHBP (6) or a fragment encompassing the RBD (amino acid residues 121–203) were expressed in the yeast three-hybrid system as AD–HBP fusions. β-Galactosidase activities were measured with extracts prepared from three independent transformants at two dilutions of extract (see Materials and Methods). Standard deviations are indicated with error bars. The values for wtHBP are the same as presented in Figure 1. (B and C) EMSA with wtHBP (B) or with the RBD (C) and short synthetic hairpin RNAs. wtHBP and a fragment encompassing the RBD (amino acid residues 121–203) were expressed in insect cells and E.coli, respectively. Both proteins contained an N-terminal histidine tag and were purified to >90% homogeneity. Protein concentrations (HBP 0.6 µM; RBD 0.2 µM) were chosen to give near complete binding of wtHP RNA. The proteins were incubated with the indicated 32P-labelled hairpin RNAs and analysed by non-denaturing gel electrophoresis and autoradiography as described in Materials and Methods. (D) Gel analysis of the free hairpin RNAs used for EMSA in (B) and (C). The additional band in the U11G RNA preparation (lane 4) that is also visible in the binding reactions is most likely due to an alternative folding of the RNA.