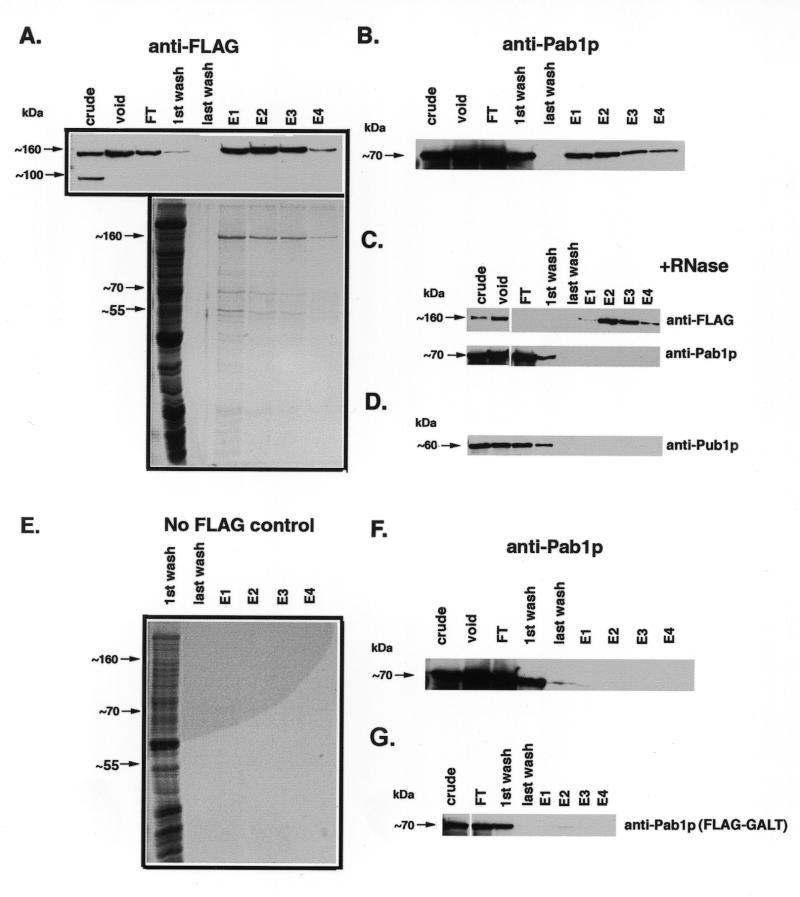
Figure 5.
α-FLAG affinity purification of Scp160p-containing complexes. Cell lysates expressing the indicated alleles of Scp160p were first passed over an S-300 gel filtration column, followed by an α-FLAG immunoaffinity column as described in the Materials and Methods section. Lanes: crude, starting material loaded onto S-300 column; void, pooled fractions containing void volume collected after S-300 run; FT, flow-through material after two passes over α-FLAG column; E1-4, 1 ml elution fractions collected after adding FLAG peptide. (A) Top, α-FLAG western blot, showing that FLAG–Scp160p appears predominantly in the first several fractions following treatment with elution buffer. Bottom, colloidal G250 Coomassie stained gel of concentrated samples. (B) α-Pab1p western, demonstrating co-isolation of Pab1p with Scp160p. (C) RNase treatment prior to α-FLAG purification disrupts the Pab1p interaction. Upper panel, α-FLAG western; lower panel, α-Pab1p western. (D) α-Pub1p western, demonstrates that this protein does not co-purify with Scp160p. (E) Negative control showing colloidal G250 Coomassie stained gel of fractions from a wild-type (non-FLAG–Scp160p) purification. (F) α-Pab1p western of negative control fractions, showing no detectable Pab1p signal. (G) Background levels of Pab1p non-specifically isolated during α-FLAG purification from yeast expressing an unrelated protein, FLAG–GALT. Since FLAG–GALT exists primarily as an 88 kDa dimer in vivo, lysates were not pre-purified over the S-300 column.