Fig. 3. The effect of tyrosine sulfation of DARC on PvDBP-RII affinity and parasite invasion.
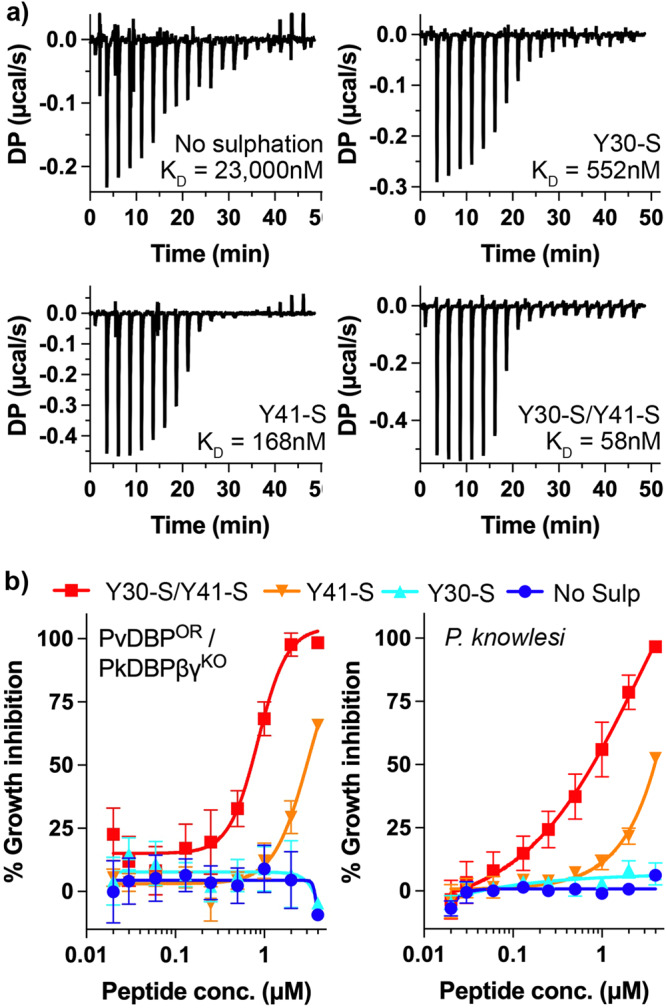
a Isothermal titration calorimetry measurements of the binding of synthetic DARC peptides to PvDBP-RII. Shown are single representative traces for non-sulfated peptide and peptides sulfated on Y30, on Y41 or on both 30 and 41. Each stated KD value is the mean from n = 3 technical replicates. b Growth-inhibitory activity for the same four peptides in an assay which assess the growth of a Plasmodium knowlesi line in which PkDBPs have been replaced by PvDBP (PvDBPOR/PkDBPβγKO, left) or wild-type Plasmodium knowlesi (right). The Y30-S/Y41-S peptide inhibited with an IC50 of 0.72 μM for PvDBPOR/PkDBPβγKO and 0.79 μM for P. knowlesi. The Y41-S peptide inhibited with an IC50 of 2.99 μM for PvDBPOR/PkDBPβγKO and 3.92 μM for P. knowlesi. Technical replicates (n = 2) from each assay were averaged, and data presented represents the mean ± standard error of the mean of four separate biological replicates (n = 2 in Fya donor blood, and n = 2 in Fyb, to account for variation between DARC alleles). IC50 values were identified using a variable slope four-parameter logistic curve. Source data are provided as a Source data file.
