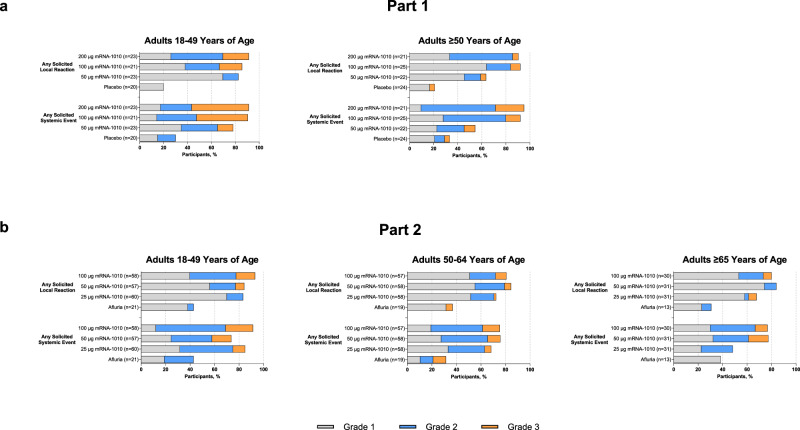
Fig. 2. Summary of any solicited local and systemic adverse reactions within 7 days after vaccination by age group in each study part.
Percentages of participants in the solicited safety population reporting any solicited adverse reactions in Part 1 (a) or Part 2 (b). In Part 1, number of participants in the placebo group were 20 (18–49 years) and 24 (≥50 years); number of participants in the mRNA-1010 groups were 23 (50 µg), 21 (100 µg), and 23 (200 µg) for 18–49 years, and 22, (50 µg), 25 (100 µg), and 21 (200 µg) for ≥50 years. In Part 2, number of participants in the Afluria group were 21 (18–49 years), 19 (50–64 years), and 13 (≥65 years); number of participants in the mRNA-1010 groups were 60 (25 µg), 57 (50 µg), and 58 (100 µg) for 18–49 years; 58 (25 µg), 58 (50 µg), and 57 (100 µg), for 50–64 years; and 31 (25 µg), 31 (50 µg), and 30 (100 µg) for ≥65 years. mRNA, messenger RNA.