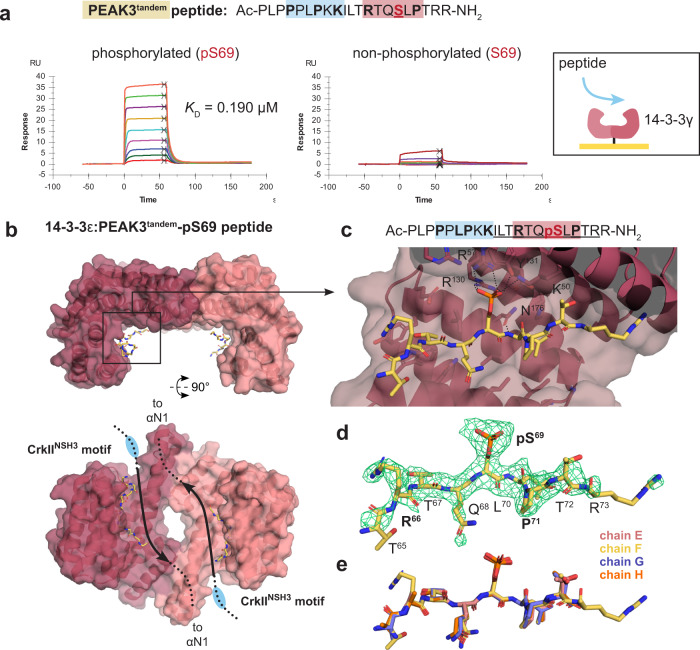
Fig. 5. Structural and biophysical analysis of PEAK3/14-3-3 interaction.
a Binding of PEAK3tandem-pS69 (left) and PEAK3tandem-S69 peptides (right) to 14-3-3ɣ as measured by SPR. b Overall structure of 14-3-3ε: PEAK3tandem-pS69 peptide showing 14-3-3 dimer antiparallel arrangement with each monomer (pink cartoon/surface) bound to a single copy of the PEAK3tandem-pS69 peptide (yellow sticks). Chain A of 14-3-3ε is in dark pink and chain B is in light pink. c Zoom in highlighting peptide groove and showing the central pS69 residue of the PEAK3 phosphopeptide interacting with K50, R57, Y131 and R130. d Unbiased Fo-Fc omit map (green mesh, contoured at 3.0σ) showing peptide density prior to modeling and final modeled PEAK3tandem-pS69 phosphopeptide (yellow sticks). e Superposition of the peptide modeled in each 14-3-3 monomer (chains A–D, sticks) in the asymmetric unit.