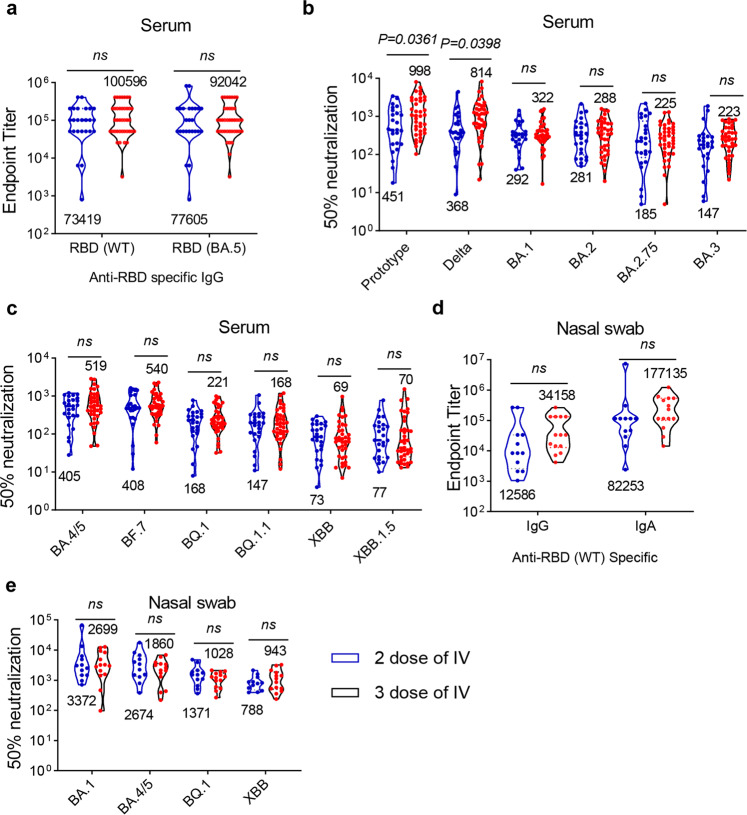
Fig. 3.
Comparison of humoral immune response after BA.5 infection with different number of vaccinations. a 64 adult (aged from 18–65) participants who have received injections of inactivated vaccines with infection were then divided into two cohorts: two doses of inactivated vaccine group (n = 25), and 3 doses of inactivated group (n = 39). The endpoint titers of wildtype and BA.5 spike-specific binding antibodies in sera were determined. b Neutralizing antibody titers against prototype, Delta, BA.1, BA.2, BA.2.75, BA.3 pseudoviruses in sera from adult participants with different number of vaccinations. c Neutralizing antibody titers to BA.4/5, BF.7, BQ.1, BQ.1.1, XBB and XBB.1.5 pseudoviruses in serum samples. (n = 25 in 2 dose of IV group, and n = 39 in 3 dose of IV group in a–c). d Endpoint titers of wildtype spike-specific binding antibodies IgG and IgG in nasal samples. e Neutralizing antibody titers to BA.1, BA.4/5, BQ.1 and XBB pseudoviruses in nasal swab samples. (n = 12 in 2 dose of IV group, and n = 14 in 3 dose of IV group in d, e). Data are presented as geometric mean values ± SD in (a–e). P values were determined by unpaired Student’s t tests. ns not significant