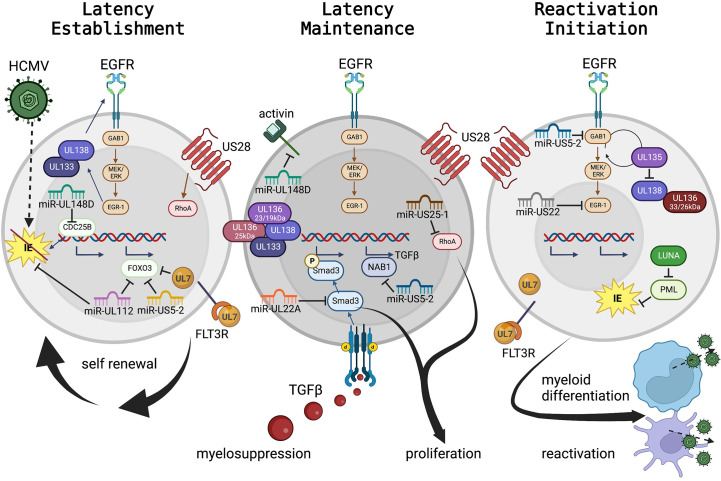
Figure 2.
HCMV regulation of progenitor cell mechanisms for latency maintenance, latency establishment, and the initiation of reactivation. HCMV viral proteins and miRNAs controlling cellular signaling, cytokine feedback loops, and as mimics of cellular receptors and ligands to control cell fate, are presented in Table 1, and select highlights are discussed here and in the text. Panel 1: Hematopoietic stem and progenitor cells (HS/HPCs) are susceptible to and the preferential site of HCMV latency establishment and maintenance. Specific viral programs regulate entry and genome delivery to the nucleus. Further viral control, including regulation of cellular receptors (including EGFR) by the UL133-UL138 genes control cellular functions (e.g. quiescence, proliferation). Additional viral products regulate signaling pathways, such as US28 regulation of RhoA and UL7 interaction with FLT3R. The viral miRNAs serve as negative regulators of cellular transcriptional pathways to control cellular genes for essential functions (e.g., miR-US5-2 and miR-UL112 regulation of FOXO3 to prevent apoptosis or miR-UL148D regulation of CDC25B to control cell cycle). Viral miRNAs also regulate viral transcription including miR-UL112 inhibition of IE to establish latency. Panel 2: Latency maintenance is also controlled by complex interactions of viral genes, proteins, miRNAs, and cellular pathways including the regulation of proliferation, cell cycle control, and cytokine production (discussed in the text and highlighted here). Latency maintenance is also accompanied by the control of the HPC extracellular environment, mediated in part by viral miRNA control of the master cell regulating cytokine, TGF-β. Panel 3: Viral reactivation and cellular differentiation to the mature myeloid lineages is a quintessential chicken and egg question – which came first? Regardless, reactivation and differentiation are inextricably linked. Viral regulation by the UL133-UL138 region triggers a shift from latency to reactivation by fine-tuning the EGFR pathway. Latency maintenance is relieved, and reactivation and differentiation are initiated by expression of specific viral proteins including US28, UL7 and LUNA; while the viral miRNAs continue to control cellular and viral transcription to regulate differentiation and proliferation which results in the production of new virus particles from mature myeloid lineage cells. (To represent the biological differences of stem cell subsets along myeloid differentiation, nuclear:cytoplasmic ratios are approximately to scale, but some cellular protein localization is solely descriptive rather than representative.).