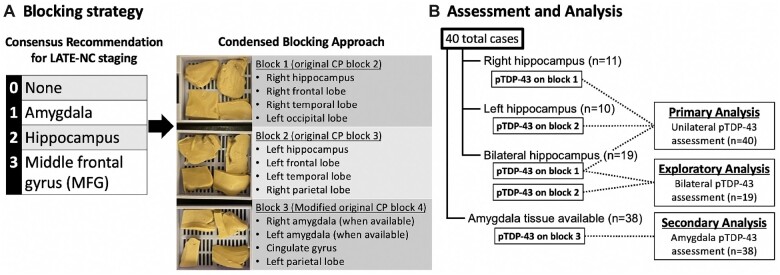
Figure 1.
(A) For this study, blocks were prepared according to the original condensed protocol for the assessment of neurodegenerative disease in order to assess the use of this condensed blocking scheme for the identification of LATE-NC compared to standard research blocking protocols. Specifically, blocks 2, 3, and 4 from the original protocol contain the brain regions relevant to the assessment of LATE-NC and were the focus of this study. Blocks 1 and 2 in the current study exactly followed the original protocol for blocks 2 and 3, respectively. Block 3 in the current study is a modified version of block 4 from the original protocol that includes bilateral amygdala sampling when available. Immunohistochemistry for pTDP-43 was applied to all 3 blocks. (B) For primary analysis, only block 1 or 2, depending on tissue availability, was included for assessment of unilateral pTDP-43 pathology in the hippocampus and frontal cortex (n = 40). In an exploratory analysis, the 19 cases with bilateral hippocampal tissue available were analyzed to determine if adding a second block significantly improved sensitivity of the CP for detecting clinically relevant LATE-NC. In a secondary analysis, the 38 cases with available amygdala were assessed to determine if the CP amygdala samples were sufficient to accurately identify LATE-NC stage 1.