Figure 5.
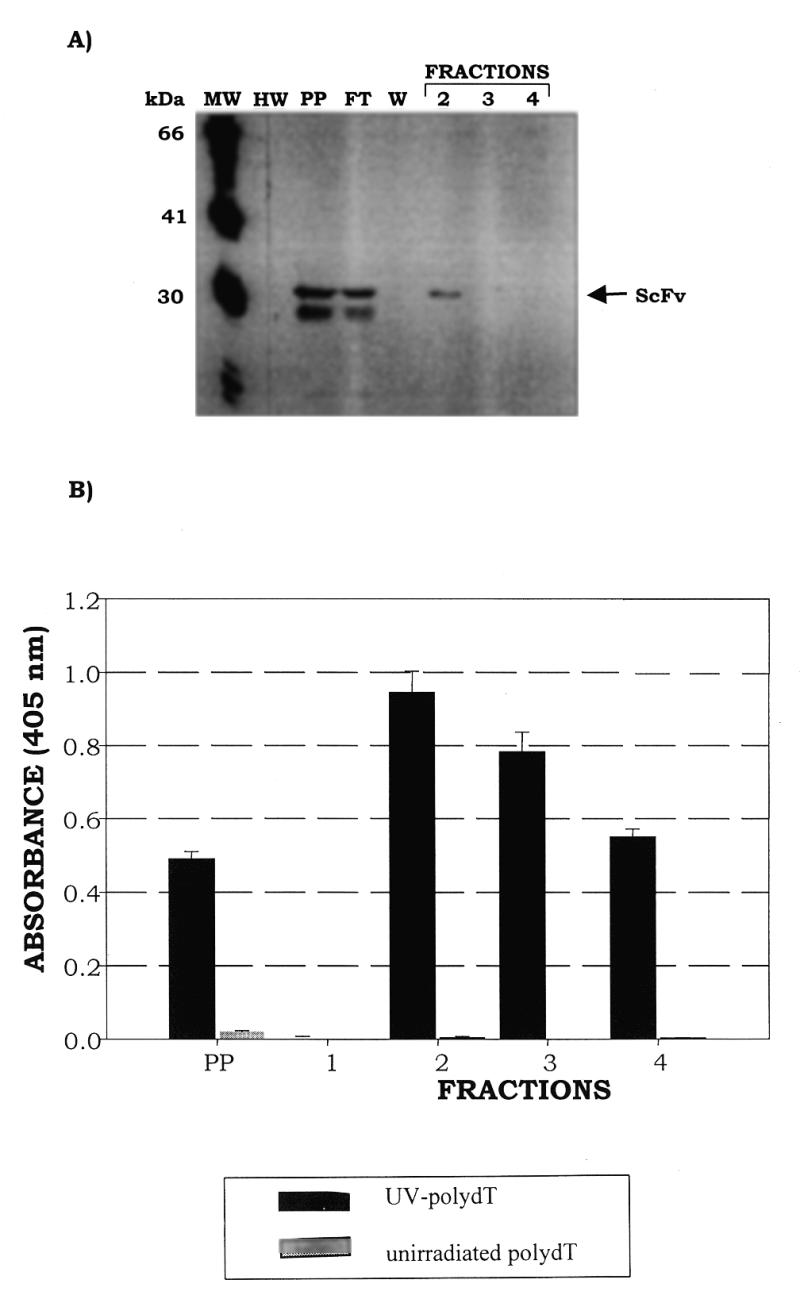
Purification of soluble C3B6 ScFv from recombinant phage clone 220-F5 using an anti-E tag/protein G affinity column. (A) Western blot of fractions eluted from the anti-E tag column; the blot was probed with anti-F(ab′)2 antibody (Jackson ImmunoResearch Laboratories). C3B6 ScFv is indicated by an arrow. Lane MW, FokI molecular weight markers; lane HW, prestained high molecular weight markers; lane PP, periplasmic extract; lane FT, flow-through; lane W, wash; lanes 2–4, fractions eluted with 1 M glycine, pH 3.0. (B) Binding activity, assessed by ELISA, of C3B6 ScFv fractions eluted from the anti-E tag column. Soluble ScFv, produced in the non-amber suppressor HB2151 strain, was passed over a protein G column to which the monoclonal anti-E tag antibody (Pharmacia Biotech) had been covalently bound. After washing with 6 column vol of 0.2 M phosphate buffer containing 0.05% NaN3 the bound protein was eluted in 1.0 M glycine, pH 3.0. ELISA plates were prepared as described in Materials and Methods and blocked for 1 h at room temperature in a solution of 5% non-fat dry milk in 0.2× PBS. Plates were then washed four times with 0.2× PBS. ScFv fractions used were diluted 1:5 to a final concentration of 2% non-fat dry milk in 0.2× PBS containing 0.02% NaN3 and 100 µl were added to four wells containing 50 ng UV-poly(dT) and four wells containing unirradiated poly(dT). Plates were incubated at 37°C with moderate shaking for 1.5 h and then washed six times with 0.2× PBS containing 0.05% Tween 20 (TPBS). Peroxidase-conjugated rabbit anti-mouse polyclonal sera with F(ab′)2 specificity (Jackson ImmunoResearch Laboratories) was diluted 1:12 000 in 2.5% non-fat dry milk in 0.2× PBS and 100 µl were added per well. Plates were incubated at 37°C with moderate shaking for 1 h and then washed six times in TPBS. Aliquots of 100 µl of ABTS substrate were added to each well and readings were taken at 405 nm after 30 min development.
