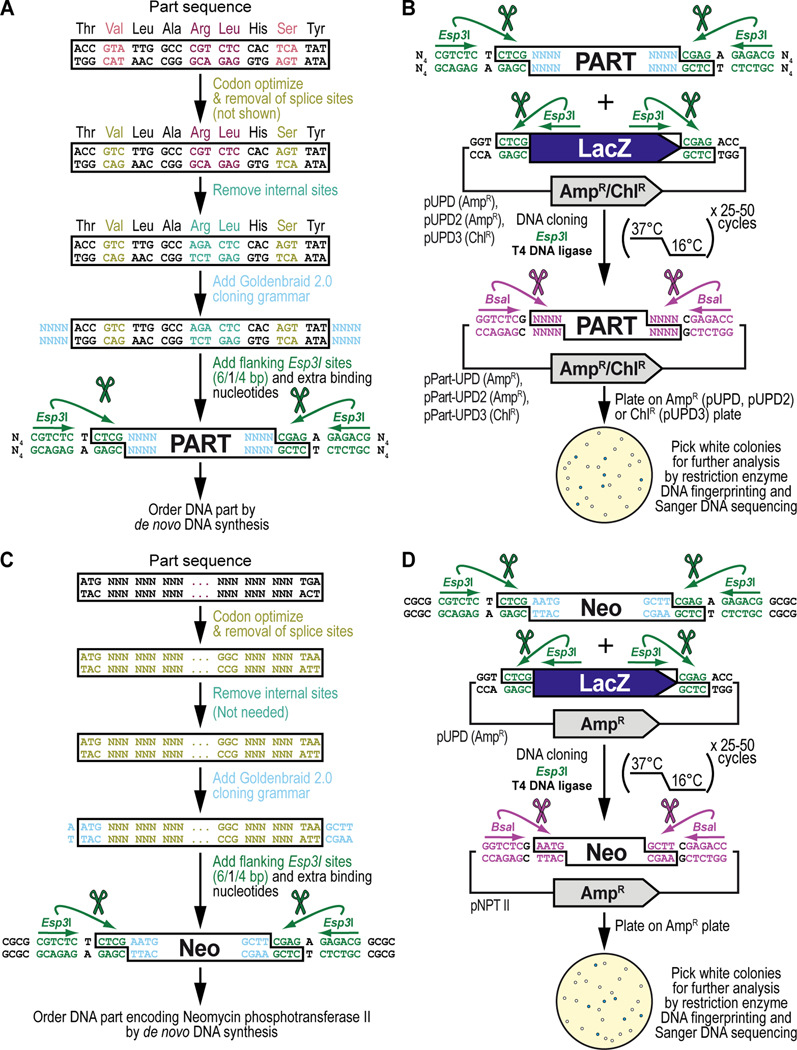
Figure 5. Obtaining and cloning a de novo synthesized DNA part for synthetic assembly cloning. (A) General principles of obtaining a DNA part by de novo DNA synthesis.
Designing the sequence for a de novo synthesized DNA part begins with codon optimization for expression in Drosophila melanogaster (shown in light green) including removal of Drosophila splice acceptor and donor sites (not shown in this example of too short of a sequence, see Text), followed by identification and manual removal of internal binding sites for the Type IIs restriction enzymes Esp3I (“CGTCTC”) (shown in cyan) and BsaI (“GGTCTC”) (not shown). Next, on either side of the DNA part, the desired 4-nucleotide Goldenbraid 2.0 cloning grammar (see Figure 2B) is added (shown in light blue), as well as a pair of inverted Esp3I sites (shown in dark green) that will generate “CTCG” overhangs required for domestication (see B). For improved restriction enzyme binding, 4 extra nucleotides, “NNNN” abbreviated to “N4” (we typically use “CGCG”) (see C), are added to the free ends of the DNA fragment as well (shown in black). An application of the principles described here are illustrated below for obtaining the DNA sequence encoding the selectable marker Neomycin phosphotransferase II (see C), used as one of the parts to build the G418 sulfate-selectable LexA transactivator plasmid (see Figure 3). (B) General principles of cloning a de novo synthesized DNA part into a pUPD vector backbone. Once synthesized, the DNA fragment is combined with a Universal Part Domesticator plasmid (pUPD, pUPD2 or pUPD3), the Type IIs restriction enzyme Esp3I, and T4 DNA ligase (including 10x T4 DNA ligase buffer). The assembly protocol cycles 25 to 50 times between 37°C (favoring cutting using Esp3I) and 16°C (favoring ligation using T4 DNA ligase). After overnight selection on bacterial plates (ampicillin for pUPD and pUPD2, or chloramphenicol for pUPD3), assembled plasmids are identified as white colonies that are characterized further by restriction enzyme DNA fingerprinting and Sanger DNA sequencing (see Text), while religated domesticator plasmids are blue due to the presence of the colorimetric LacZ α-fragment. An application of the principles described here is illustrated below for cloning of the DNA part encoding the selectable marker Neomycin phosphotransferase II (see D), used as one of the parts to build the G418 sulfate-selectable LexA transactivator plasmid (see Figure 3). (C) DNA synthesis of the DNA part encoding Neomycin phosphotransferase II (Neo). Designing the DNA part encoding Neomycin phosphotransferase II (Neo) begins with codon optimization for expression in Drosophila melanogaster (shown in light green) including removal of Drosophila splice acceptor and donor sites (see Text), followed by identification and manual removal of internal binding sites for the Type IIs restriction enzymes Esp3I (CGTCTC) and BsaI (GGTCTC) (both not needed in this example). Next, on either side of the DNA part, the 5’ “AATG” and 3’ “GCTT” Goldenbraid 2.0 cloning grammars are integrated/added (shown in light blue) to couple it to a 5’UTR and 3’UTR, respectively (see Figure 2B), as well as a pair of inverted Esp3I sites (shown in dark green) that will generate overhangs (CTCG) required for domestication (see D). For improved restriction enzyme binding, the tetranucleotide “CGCG” is added last (shown in black). (D) Cloning of the DNA part encoding Neomycin phosphotransferase II (Neo) into pUDP. Once synthesized, the “Neo” fragment is combined with the Universal Part Domesticator plasmid pUPD, the Type IIs restriction enzyme Esp3I, and T4 DNA ligase (including 10x T4 DNA ligase buffer). The assembly protocol cycles 25 to 50 times between 37°C (favoring cutting using Esp3I) and 16°C (favoring ligation using 10x T4 DNA ligase buffer). After overnight selection on bacterial plates supplemented with ampicillin for pUPD, assembled plasmids are identified as white colonies that are characterized further by restriction enzyme DNA fingerprinting and Sanger DNA sequencing (see Text), while religated domesticator plasmids are blue due to the presence of the colorimetric LacZ α-fragment.