Figure 2. NF-κB activity of flavopiridol-loaded microparticles (FPs).
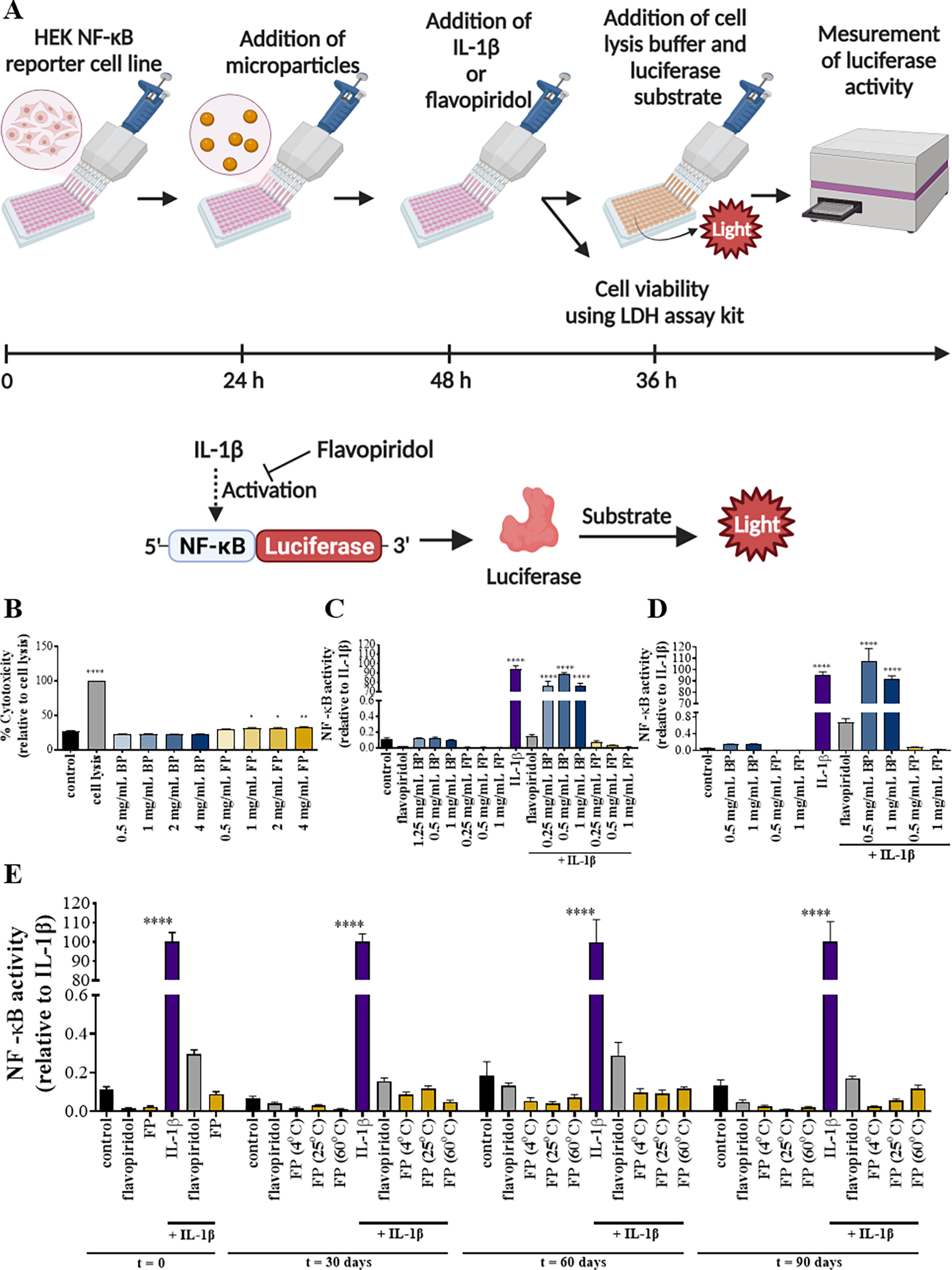
Methods used to determine the viability and bioactivity of FPs (A). Cytotoxicity of PLGA particles encapsulating flavopiridol was evaluated (B). NF-κB luciferase activity of PLGA particles encapsulated with flavopiridol in experiment settings performed in a regular plate (C) or in a transwell plate (D). Reporter cells were treated with media, soluble flavopiridol, IL-1β, blank MPs and/or flavopiridol-loaded MPs and the supernatants were collected and analyzed for NF-κB luciferase activity. For stability of PLGA particles encapsulating flavopiridol (E), NF-κB luciferase activity of microparticles loaded with flavopiridol after storage at different temperatures for different time periods 0, 30, 60, and 90 days was examined. Data are presented as the mean ± S.E. (n ≥ 3 independent experiments). The statistically significant differences were calculated in comparison to the control where the following symbols represent the stated levels of significance: * - p<0.05, ** - p<0.01, and **** - p<0.0001 as determined by a one-way analysis of variance (ANOVA), followed by Tukey’s multiple-comparison test.
