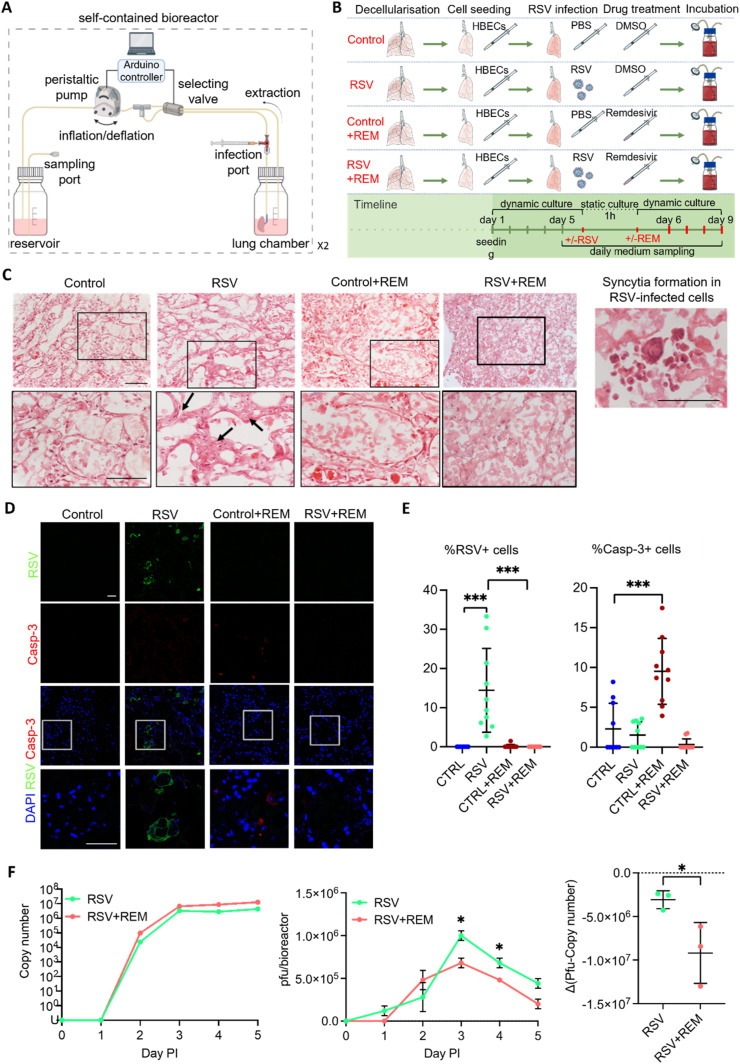
Fig. 5.
Engineered lung infection. A. Schematic of the bioreactor setup for the engineered lung infection and medium sampling. This includes the addition of ports for the safe injection of the viral suspension into the lung and the retrieval of virus-contaminated supernatants. B. Experimental setup for the recellularization and infection of the engineered lung with an RSV virus in the presence or absence of the antiviral drug remdesivir. A non-infection condition and a treatment with remdesivir without RSV infection were included as controls. C. Haematoxylin and eosin (H&E) analysis of the engineered lungs cultured under the four experimental conditions. Black arrows show a thickened parenchyma in the RSV condition that were observed in the higher magnification pictures (row below). Scale bar = 100 μm. D. Immunofluorescence analysis of RSV and cleaved caspase-3 proteins in engineered lungs cultured under the four experimental conditions showed RSV-positive cells in the infected lung with no remdesivir and the presence of apoptotic cells (Casp-3). Scale bar = 50 μm. E. Quantification of the immunofluorescence analysis in D shows a significant increase in the percentage of RSV-positive cells in the RSV infection condition (left). The percentage of Caspase 3-positive cells is significantly increased in engineered lungs cultured with remdesivir only. T-test; ***p < 0.005. F. Analysis of the viral titer of the supernatants collected from the RSV-infected engineered lungs without or with remdesivir, at different days post infection (PI). Quantitative RT-PCR of RSV viral load in media samples measured by N protein copy number. Infectious particles measured via Plaque-forming unit (pfu) assay, calculated for all circulating media within a bioreactor (80 mls) (pfu/bioreactor) (central). The difference between pfu and copy number (right). Points show mean ± SD, n = 3. Multiple t-test; *p < 0.05.