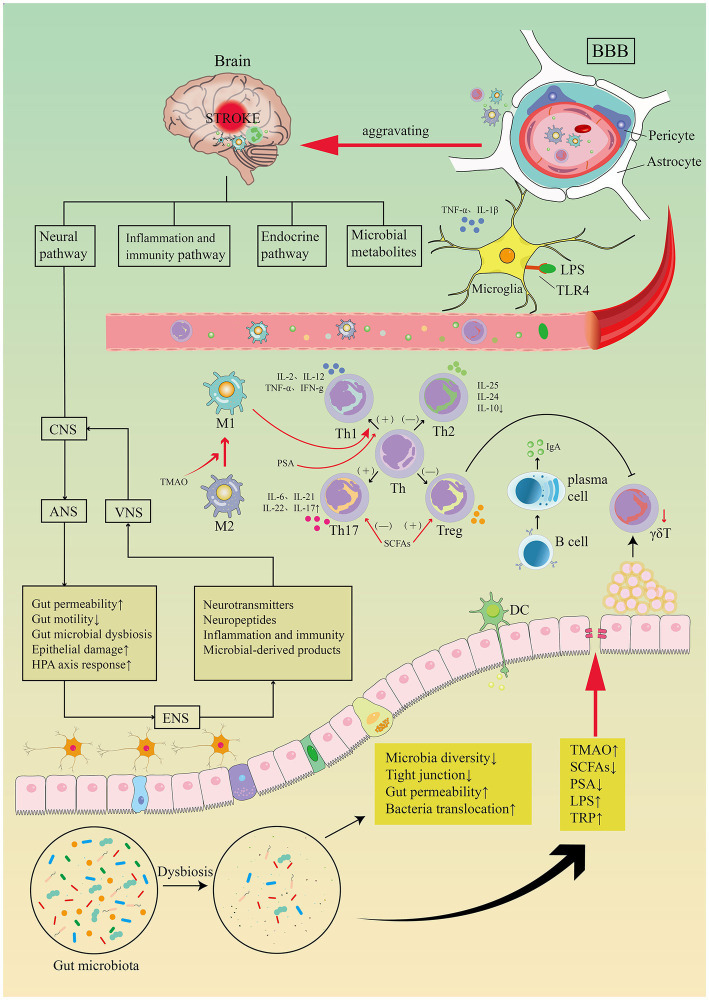
Figure 8.
Image for potential association pathways between gut flora and stroke. The first is inflammatory and immune pathways. Stroke causes dysbiosis of the intestinal flora and decreased species diversity, impaired tight junctions and bacterial translocation, increased bacterial metabolites TMAO, LPS, TRP, decreased SCFAs, PSA. Intestinal flora regulates CD4+ T cell differentiation to CD8+ T cells via epithelial or DC cell-mediated signaling. T cell subsets can differentiate toward helper T cells (e.g., Th1, Th2, Th17) and regulatory T cells (e.g., Treg), the dysbiosis of the gut flora may trigger pro-inflammatory Th1 and Th17 Thelper cell polarization. Reduced manufacturing of γδT lymphocytes results in intestinal-to-membrane transfer, resulting to increased chemotactic factor production and brain infiltration of cytotoxic cells. Treg cells contribute in the post-stroke immunological cascade by inhibiting IL-17T cell proliferation via an intestinal route. SCFAs induce differentiation of naive T lymphocytes toward a functional Treg phenotype and suppress the pro-inflammatory Th17 phenotype, modulate microglia activation, and regulate synaptic plasticity after stroke. PSA also regulates differentiation of primitive CD4+ T cells toward Th1 cells, skewing the Th1/Th2 ratio in favor of Th1 cells. LPS stimulates Toll-like receptor 4 in brain microglia, astrocytes, and neurons, activating downstream immune responses and boosting the production of pro-inflammatory chemicals. TMAO enhances the polarization of M1 macrophages and the differentiation of Th cells into Th1 and Th17 subsets. Top-down neurotransmission mechanism: the sympathetic and parasympathetic nervous systems in the autonomic nervous system (ANS) integrate various messages from the brain, which are then transmitted to the enteric nervous system (ENS) or directly to the intestinal wall, resulting in necrosis and shedding of intestinal epithelial cells, increased intestinal permeability, impaired intestinal motility, and dysbiosis of the intestinal flora, HPA axis activation, and dysbiosis of the intestinal. Bottom-up neurotransmission mechanisms: a series of responses following intestinal flora disorders are perceived by the enteric nervous system (ENS) and reach the nucleus tractus solitarius (NTS) via sensory fiber inputs in the vagus nerve, which are then transmitted to extensive regions of the central nervous system for interpretation. Th, T helper; Treg, T regulatory; TMAO, trimethylamine-N-oxide; SCFAs, short-chain fatty acids; PSA, polysaccharide A; LPS, lipopolysaccharide; TRP, tryptophan.