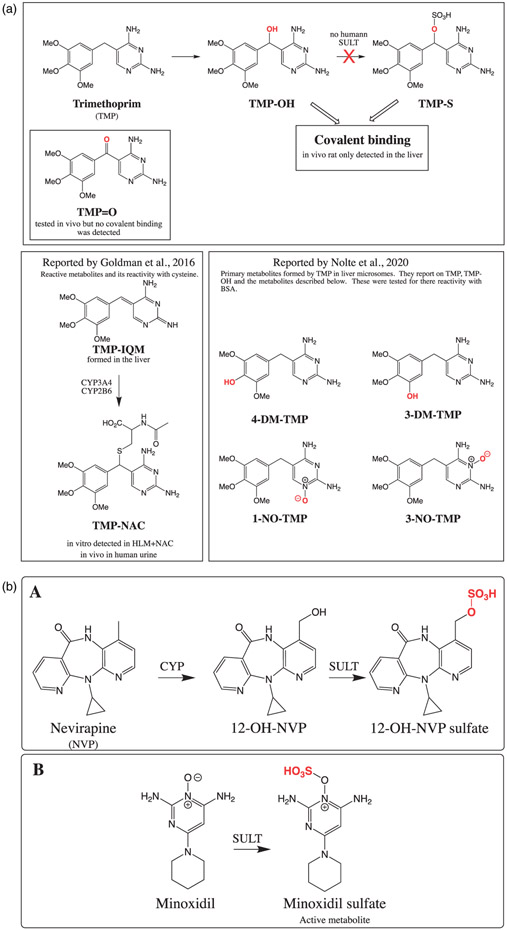
Figure 6.
(a) Overall reported trimethoprim (TMP) and its reactive metabolites. In vivo rat studies, TMP formed significant hepatic covalent binding but not with α-hydroxy-TMP (TMP-OH) and α-keto-TMP (TMP = O). In vitro TMP-OH and TMP-S formed covalent binding in S9 fractions with liver ⪢ skin. Finally, no human SULT was found to convert TMP-OH to TMP-S. In the two lower boxes strucutres of TMP reactive metabolites and primary metabolites reported by Goldman et al. 2016 and Nolte et al. 2020. (b) (A) The reaction for the nevirapine (NVP) CYP-mediated metabolism to 12-hydroxy NVP (12-OH-NVP) and its subsequent metabolism by human sulfotransferases (SULT). (B) Minoxidil formation of pharmacologically active minoxidil sulfate.