Abstract
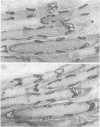
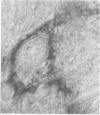
Anti-Leu 7 monoclonal antibody (MAB), a marker of natural killer cells, and a human MAB to myelin-associated glycoprotein (MAG) from a patient with a demyelinating neuropathy specifically stained Schmidt-Lanterman incisures, paranodal and periaxonal regions in peripheral nerve myelin by immunocytochemistry on thin plastic sections, while compact myelin was labelled in paraffin-embedded material. Preabsorption studies indicated that the antigen recognised was a MAG epitope shared by MAG and Leu 7. In spinal cord both MABS bound to oligodendrocytes and a subclass of anterior horn cells. Our findings support the hypothesis that shared antigens between the nervous and the immune systems do exist in situ, which may be important in the pathogenesis of demyelinating neuropathies with monoclonal gammopathies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Cooper M. D., Balch C. M. Postnatal expansion of the natural killer and keller cell population in humans identified by the monoclonal HNK-1 antibody. J Exp Med. 1982 Jan 1;155(1):321–326. doi: 10.1084/jem.155.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams G. M., Latov N., Hays A. P., Sherman W., Zimmerman E. A. Immunocytochemical studies of human peripheral nerve with serum from patients with polyneuropathy and paraproteinemia. Neurology. 1982 Aug;32(8):821–826. doi: 10.1212/wnl.32.8.821. [DOI] [PubMed] [Google Scholar]
- Abruzzo L. V., Rowley D. A. Homeostasis of the antibody response: immunoregulation by NK cells. Science. 1983 Nov 11;222(4624):581–585. doi: 10.1126/science.6685343. [DOI] [PubMed] [Google Scholar]
- Braun P. E., Frail D. E., Latov N. Myelin-associated glycoprotein is the antigen for a monoclonal IgM in polyneuropathy. J Neurochem. 1982 Nov;39(5):1261–1265. doi: 10.1111/j.1471-4159.1982.tb12563.x. [DOI] [PubMed] [Google Scholar]
- Denton C. P., Gregson N. A., Leibowitz S., Kennedy M. The binding of human IgM paraprotein from cases of polyneuropathy associated with benign monoclonal gammopathy to specific neurons of the rodent brain. Brain Res. 1984 Mar 19;295(2):348–351. doi: 10.1016/0006-8993(84)90983-1. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Ree H. J. Self-sandwich method. An improved immunoperoxidase technic for the detection of small amounts of antigens. Am J Clin Pathol. 1980 Jul;74(1):32–40. doi: 10.1093/ajcp/74.1.32. [DOI] [PubMed] [Google Scholar]
- Ilyas A. A., Quarles R. H., MacIntosh T. D., Dobersen M. J., Trapp B. D., Dalakas M. C., Brady R. O. IgM in a human neuropathy related to paraproteinemia binds to a carbohydrate determinant in the myelin-associated glycoprotein and to a ganglioside. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1225–1229. doi: 10.1073/pnas.81.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry R. C., Helfand S. L., Quarles R. H., Roder J. C. Recognition of myelin-associated glycoprotein by the monoclonal antibody HNK-1. Nature. 1983 Nov 24;306(5941):376–378. doi: 10.1038/306376a0. [DOI] [PubMed] [Google Scholar]
- Murray N., Steck A. J. Indication of a possible role in a demyelinating neuropathy for an antigen shared between myelin and NK cells. Lancet. 1984 Mar 31;1(8379):711–713. doi: 10.1016/s0140-6736(84)92224-4. [DOI] [PubMed] [Google Scholar]
- Quarles R. H., Pasnak C. F. A rapid procedure for selectively isolating the major glycoprotein from purified rat brain myelin. Biochem J. 1977 Jun 1;163(3):635–637. doi: 10.1042/bj1630635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner M. Cell type-specific surface antigens in the mammalian nervous system. J Neurochem. 1982 Jul;39(1):1–8. doi: 10.1111/j.1471-4159.1982.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Schuller-Petrovic S., Gebhart W., Lassmann H., Rumpold H., Kraft D. A shared antigenic determinant between natural killer cells and nervous tissue. Nature. 1983 Nov 10;306(5939):179–181. doi: 10.1038/306179a0. [DOI] [PubMed] [Google Scholar]
- Steck A. J., Murray N., Meier C., Page N., Perruisseau G. Demyelinating neuropathy and monoclonal IgM antibody to myelin-associated glycoprotein. Neurology. 1983 Jan;33(1):19–23. doi: 10.1212/wnl.33.1.19. [DOI] [PubMed] [Google Scholar]
- Steck A. J., Murray N., Vandevelde M., Zurbriggen A. Human monoclonal antibodies to myelin-associated glycoprotein. Comparison of specificity and use for immunocytochemical localisation of the antigen. J Neuroimmunol. 1983 Oct;5(2):145–156. doi: 10.1016/0165-5728(83)90005-x. [DOI] [PubMed] [Google Scholar]
- Sternberger N. H., Quarles R. H., Itoyama Y., Webster H. D. Myelin-associated glycoprotein demonstrated immunocytochemically in myelin and myelin-forming cells of developing rat. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1510–1514. doi: 10.1073/pnas.76.3.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B. D., Quarles R. H. Presence of the myelin-associated glycoprotein correlates with alterations in the periodicity of peripheral myelin. J Cell Biol. 1982 Mar;92(3):877–882. doi: 10.1083/jcb.92.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]