Abstract
Aims
Transcatheter aortic valve implantation (TAVI) is the treatment of choice for high-risk patients with severe aortic stenosis (AS). A portion of TAVI recipients has no long-term clinical benefit, and myocardial fibrosis may contribute to unfavourable outcomes. We aimed to assess the prognostic value of an interstitial fibrosis marker, extracellular volume fraction (ECV), measured at planning computed tomography (CT) before TAVI.
Methods and results
From October 2020 to July 2021, 159 consecutive patients undergoing TAVI planning CT were prospectively enroled. ECV was calculated as the ratio of myocardium and blood pool differential attenuations before and 5 min after contrast administration, pondered for haematocrit. A composite endpoint including heart failure hospitalization (HFH) and death was collected by telehealth or in-person follow-up visits in the 113 patients constituting the final study population. Cox proportional hazards model was used to assess association between ECV and the composite endpoint.
Median follow-up was 13 (11–15) months. The composite endpoint occurred in 23/113 (20%) patients. These patients had lower aortic valve mean pressure gradient [39 (29–48) vs. 46 (40–54) mmHg, P = 0.002] and left ventricular and right ventricular ejection fraction [51 (37–69) vs. 66 (54–74)%, P = 0.014; 45 (31–53) vs. 49 (44–55)%, P = 0.010] and higher ECV [31.5 (26.9–34.3) vs. 27.8 (25.3–30.2)%, P = 0.006]. At multivariable Cox analysis, ECV higher than 31.3% was associated to increased risk of death or HFH at follow-up (hazard ratio = 5.92, 95% confidence interval 2.37–14.75, P < 0.001).
Conclusion
In this prospective observational cohort study, ECV measured at TAVI planning CT predicts the composite endpoint (HFH or death) in high-risk severe AS patients.
Keywords: TAVI, TAVR, aortic stenosis, ECV, CT
Graphical Abstract
Graphical Abstract.
Introduction
Aortic stenosis (AS) is the most common primary valve disease in the developed countries.1 AS causes progressive left ventricular (LV) pressure overload2 and consequent remodelling with myocyte hypertrophy and myocardial fibrosis.3 The natural history of untreated severe AS involves progressive LV systolic dysfunction, leading to heart failure and ultimately death.4 In older and high surgical risk patients, transcatheter aortic valve implantation (TAVI) is the treatment of choice.5 However, elder patients are also at higher risk of having developed severe irreversible LV remodelling, explaining why a significant portion of patients does not improve after TAVI.6 Cardiac magnetic resonance (CMR) studies have shown that focal replacement fibrosis7–9 and reactive interstitial fibrosis10,11 are associated with poor prognosis in patients with AS.
Recently, cardiac computed tomography (CCT) has emerged as an alternative tool to CMR for the evaluation of both focal replacement fibrosis12–14 and reactive interstitial fibrosis15–18 through extracellular volume fraction (ECV) quantification.
ECV can be easily quantified by adding a low-dose late-phase scan in the planning cardiac CT exam mandatory before TAVI for device sizing and access route assessment.19 Thus, we aimed to assess the prognostic value of ECV derived from TAVI planning CT, in patients with severe AS undergoing TAVI.
Methods
Patient population
This was an observational prospective cohort study performed at a single tertiary care university hospital. The study, approved by the institutional review board (CT-based myocardial characterization study: CTMyoC 112/INT/2019), was conducted according to the Declaration of Helsinki, and written informed consent was obtained for all participants.
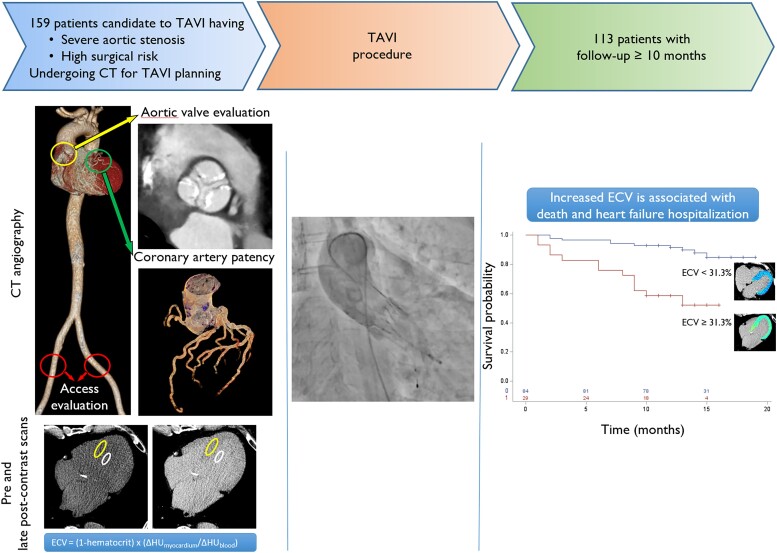
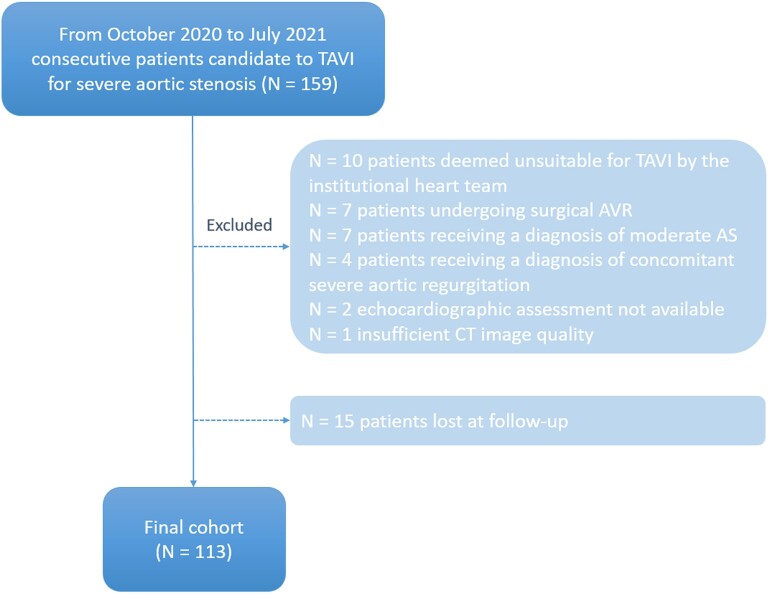
From October 2020 to July 2021, 159 consecutive participants undergoing CT for TAVI planning were enrolled according to the following inclusion criteria: (i) diagnosis of severe AS, (ii) candidate to TAVI for high surgical risk, and (iii) life expectancy >1 year. Among these patients, 31 were excluded from the study because of not undergoing TAVI, inadequate CT image quality, or ultrasound (US) unavailability (Figure 1). Fifteen patients were lost at follow-up and were excluded from the study, bringing the total population to 113 patients (Figure 1).
Figure 1.
Patient enrollment flowchart. TAVI, transcatheter aortic valve implantation; AVR, aortic valve replacement; AS, aortic stenosis; CT, computed tomography.
Pre-operative echocardiography was performed by experienced cardiologists.
Demographic information, medical history, and laboratory values were extracted from electronic medical records.
TAVI was performed via a subclavian or transfemoral approach using self-expandable or balloon-expandable devices, according to patients’ characteristics.
Echocardiography protocol
The transthoracic echocardiograms were performed at rest with commercially available US systems [Vivid E95 GE (General Electric Healthcare, Milwaukee, WI, USA) with MS5-D probe; Philips EPIQ7 (Philips Electronics, Amsterdam, The Netherlands) with X5 probe].
A complete 2D, colour, pulsed, and continuous wave Doppler echocardiogram was performed according to EACVI recommendations.20
Images were analysed off-line on a dedicated workstation, using Suitestensa CVIS (Ebit, Esaote). In the presence of severe AS with aortic valve area (AVA) <1 cm2, patients with mean aortic pressure gradient >40 mmHg were classified as high gradient (HG), while patients with aortic mean pressure gradient <40 mmHg and stroke volume indexed <35 mL/m2 were defined as low-flow low-gradient (LF-LG).20
CT protocol
CT scans were performed on a second-generation dual-source scanner (SOMATOM Definition Flash, Siemens Healthcare, Erlangen, Germany). Cardiac pre-contrast and late post-contrast scans were performed using a prospective electrocardiogram (ECG)-gated scan at fixed 280 ms delay from R-wave. CT angiography (CTA) was performed using a retrospective ECG-gated cardiac scan, immediately (5 s delay) followed by an ultra-high pitch thoracic–abdominal scan, for device sizing, valvular and coronary assessment, and vascular access route evaluation. Cardiac CTA was performed with bolus triggering in the left ventricle during injection of iodinated contrast media, Visipaque 320 (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Contrast bolus was tailored to patients’ size [85 mL for body mass index (BMI) <25; 95 mL for BMI 25–30; and 110 mL for BMI >30].
CT post-processing
Pre- and post-contrast CT scans were reconstructed with a field of view limited to the heart, slice thickness of 3 mm without overlap, smooth kernel, and no iterative reconstruction. Cardiac CTA for coronary artery evaluation was reconstructed at end-diastolic and end-systolic phases, slice thickness of 0.625 mm with an overlap of 0.125 mm, intermediate kernel, and iterative reconstruction. Multiphase (every 5% of R–R interval) cardiac CTA for TAVI planning and for quantification of LV and right ventricular (RV) volumes and ejection fraction (EF), mass, and AVA was reconstructed with a slice thickness of 1 mm with an overlap of 0.2 mm, intermediate kernel, and iterative reconstruction.
Aortic valve calcium score and coronary artery calcium score from pre-contrast CT scan were extracted using a commercially available software (IntelliSpace Portal v.10, Philips, The Netherlands). LV and RV end-diastolic (EDV) and end-systolic (ESV) volumes and EF, LV myocardial mass, and aortic root dimensions for TAVI planning were quantified from cardiac CTA using the same software. CT-derived AVA was measured with multiplanar reconstruction of the AV orifice in minimum intensity projection with a thickness of 10 mm. When appropriate, parameters were indexed to body surface area (BSA).
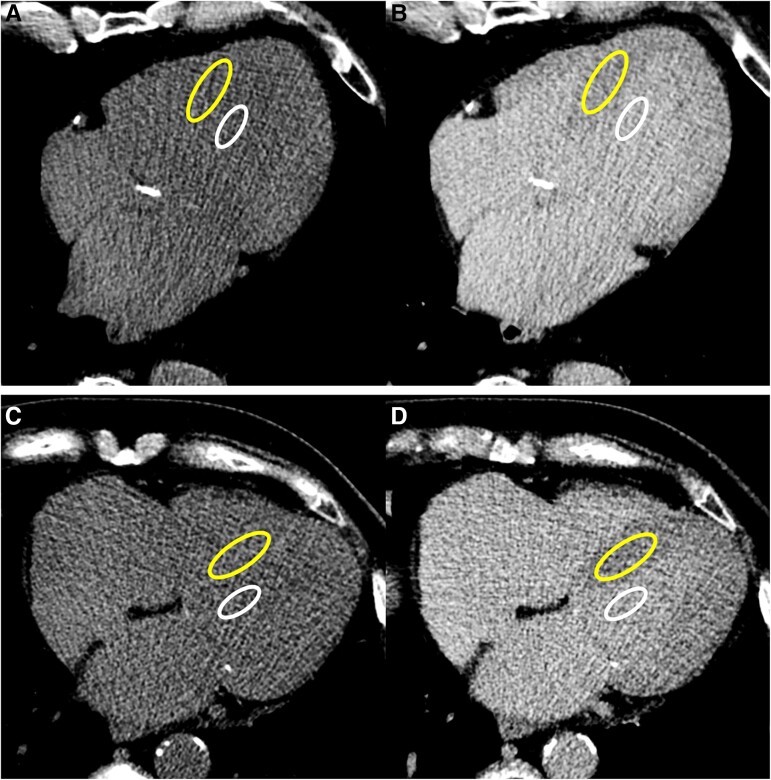
For ECV quantification, post-contrast CT attenuation in Hounsfield units (HU) of the myocardium was determined by drawing a region of interest (ROI) >2 cm2 on the interventricular septum on a mid-ventricular slice free of beam hardening or streak artefacts and of myocardial scars. Post-contrast CT attenuation of blood pool was determined by drawing a ROI >1 cm2 in the LV blood pool on the same slice. Using the picture archiving and communicating system (Enterprise Imaging, Agfa HealthCare, Belgium), pre- and post-contrast CT scans were rigidly coregistered, and myocardium and blood pool ROIs were copied to the pre-contrast scan (Figure 2). ECV was calculated according to the following formula: ECVCT = (1 − haematocrit) × (ΔHUmyo/ΔHUblood), where ΔHUmyo and ΔHUblood represent the differential attenuation of myocardium and blood pool before and after contrast administration. The mass of interstitial fibrosis was calculated as ECV multiplied for LV mass.
Figure 2.
ECV measurement in two exemplificative cases. Regions of interest (ROIs) were drawn in the LV blood pool (smaller ROI in white) and in the mid-ventricular septum (larger ROI in yellow) on an axial slice in the late post-contrast scan (B and D) and were then copied on the coregistered pre-contrast scan (A and C). Top row images show the case of an 85-year-old male with an ECV of 37.3% who experienced heart failure hospitalization (HFH) 14 months after TAVI. Bottom row images show the case of a 78-year-old female with an ECV of 25.3% who has not experienced death of HFH 17 months after TAVI.
Analyses were performed by a reader with 7 years of experience in cardiovascular imaging. ECV was measured also by a second reader with 3 years of experience to assess inter-reader variability.
Study endpoint
The primary endpoint was a composite of all-cause mortality and heart failure hospitalization (HFH) assessed during a follow-up period of at least 10 months post-TAVI. Follow-up data were obtained with telehealth or in-person medical visits. For each patient reaching the adverse outcome, the first event (death or HFH) served as the endpoint.
Statistical analysis
Continuous variables are presented as median and interquartile ranges (IQR) and categorical variables as absolute values and percentages. The Youden index was used to obtain a cut-off that optimized the differentiation of ECV. The comparison between groups was performed with Mann–Whitney U test or χ2 test as appropriate. Correlation among variables was evaluated with Spearman’s rank correlation. The association between ECV and combined endpoint (death or HFH) was assessed by Kaplan–Meier analysis, and the groups were compared by log-rank test. To investigate which variables could be associated with the combined endpoint, univariate and forward stepwise Cox proportional hazards models were used to estimate Cox hazard ratios.
All tests were two-tailed and a P-value <0.05 was required for statistical significance. All calculations were computed using SAS software package (Version 9.4, SAS Institute Inc., Cary, NC, USA).
Radiation exposure
Mean dose length product (DPL) of the whole CT examination was 1828 ± 511 mGy/cm. The mean DLP of the late scan for ECV evaluation was 86 ± 15 mGy/cm, accounting for 4.7% of the total radiation exposure.
Results
Patients’ characteristics
In the final population of 113 patients, after a median follow-up of 13 (11–15) months, the primary endpoint occurred in 23/113 (20%), including 12 deaths and 11 HFHs.
Patients were mostly female (59/113, 52%) with a median age of 82 (79–85) years and a BMI of 25.2 (22.6–27.7). B-type natriuretic peptide (BNP) and troponin T (TnT) values were 21 (16–34) ng/dL and 1101 (478–2872) ng/L, respectively, and 12/113 (27%) patients had history of previous revascularization. Details about cardiovascular risk factors and co-morbidities are summarized in Table 1.
Table 1.
Clinical data and imaging parameters in the whole population and divided according to the outcome
| Whole population (n = 113) | Patients without death or HFH (n = 90) | Patients with death or HFH (n = 23) | P-value | |
|---|---|---|---|---|
| Age (years) | 82 (79–85) | 82 (79–85) | 85 (79–88) | 0.096 |
| Male sex (%) | 54 (48) | 43 (48) | 11 (48) | 0.997 |
| BMI (kg/m2) | 25.2 (22.6–27.7) | 25.3 (22.6–28.0) | 24.7 (22.3–26.0) | 0.293 |
| Diabetes (%) | 36 (32) | 29 (32) | 7 (30) | 0.870 |
| Hypertension (%) | 87 (77) | 69 (77) | 18 (78) | 0.314 |
| Hypercholesterolaemia (%) | 70 (62) | 61 (68) | 9 (39) | 0.012 |
| Smoking history (%) | 29 (26) | 21 (23) | 8 (35) | 0.262 |
| CKD (%) | 27 (24) | 21 (23) | 6 (26) | 0.782 |
| Previous revascularization (%) | 30 (27) | 26 (29) | 4 (17) | 0.305 |
| PCI | 21 (19) | 18 (20) | 3 (13) | |
| CABG | 6 (5) | 5 (6) | 1 (4) | |
| Both | 3 (3) | 3 (3) | 0 (0) | |
| LF-LG aortic stenosis | 15 (13) | 8 (9) | 7 (30) | 0.007 |
| BNP (ng/dL) (n = 62) | 1101 (479–2872) | 1039 (479–2437) | 1546 (646–7384) | 0.273 |
| TnT (ng/L) (n = 101) | 21 (16–34) | 21 (16–31) | 26 (15–41) | 0.261 |
| Echocardiographic measurements | ||||
| LVEF (%) | 60 (56–64) | 60 (57–65) | 59 (51–63) | 0.062 |
| AVA indexed (cm2/m2) | 0.41 (0.35–0.50) | 0.41 (0.35–0.52) | 0.41 (0.34–0.47) | 0.512 |
| AV mean gradient (mmHg) | 44 (40–53) | 46 (40–54) | 39 (29–48) | 0.002 |
| IVS thickness (mm) | 13 (12–14) | 13 (12–14) | 12 (11–14) | 0.079 |
| LVPW thickness (mm) | 11 (10–12) | 11 (10–12) | 11 (10–12) | 0.237 |
| Relative wall thickness (mm) | 0.53 (0.46–0.60) | 0.53 (0.46–0.61) | 0.50 (0.45–0.58) | 0.407 |
| CT measurements | ||||
| LV-EDV indexed (mL/m2) | 78 (70–95) | 77 (68–92) | 92 (70–108) | 0.084 |
| LVEF (%) | 65 (49–74) | 66 (54–74) | 51 (37–69) | 0.014 |
| LV mass indexed (g/m2) | 84 (72–100) | 84 (73–97) | 83 (69–117) | 0.770 |
| RV-EDV indexed (mL/m2) | 85 (72–95) | 81 (70–93) | 88 (80–109) | 0.050 |
| RV-EF (%) | 47 (42–54) | 49 (44–55) | 45 (31–52) | 0.010 |
| AV calcium score (AU) | 2180 (1489–3332) | 2383 (1569–3370) | 1692 (1261–2882) | 0.074 |
| AVA indexed (cm2/m2) | 0.40 (0.35–0.49) | 0.40 (0.35–0.48) | 0.41 (0.36–0.59) | 0.169 |
| CACS (AU) | 749 (235–1612) | 866 (274–1593) | 613 (154–1737) | 0.757 |
| ECV (%) | 28.4 (25.8–31.3) | 27.8 (25.3–30.2) | 31.5 (26.9–34.3) | 0.006 |
| Fibrosis mass indexed (g/m2) | 23.9 (19.4–30.8) | 23.7 (19.3–29.8) | 27.9 (19.9–37.5) | 0.134 |
P-values less than 0.05 are depicted in bold.BMI, body mass index; CKD, chronic kidney disease; PCI, percutaneous coronary intervention; CABG, coronary artery by-pass graft; LG-LG, low-flow low-gradient; BNP, brain natriuretic peptide; TnT, troponin-T; LVEF, left ventricular ejection fraction; AVA, aortic valve area; IVS, interventricular septum; LVPW, left ventricular posterior wall; EDV, end-diastolic volume; CACS, coronary artery calcium score; ECV, extracellular volume fraction.
At echocardiography, LVEF was 60 (56–64) %, aortic valve area indexed (AVAi) 0.41 [0.35–0.50] cm2/m2, and mean AV pressure gradient 44 (40–53) mmHg. HG severe AS was diagnosed in 98/113 (87%), while the remaining 15/113 (13%) patients had LF–LG severe AS.
At CT, AV calcium score was 2180 (1489–3332) AU, AVAi 0.40 (0.35–0.49) cm2/m2, and ECV 28.4 (25.8–31.3) %. Other US and CT parameters are summarized in Table 1.
Comparison between patients experiencing or not the composite endpoint
Patients experiencing HFH or death at follow-up had more frequently LF–LG severe AS [8/23 (30%) vs. 7/90 (9%), P = 0.007] and lower AV mean pressure gradient [39 (29–48) vs. 46 (40–54) mmHg, P = 0.002]. No significant differences were noted for age, sex, BMI, BNP, and TnT (Table 1).
At CT, patients experiencing the composite endpoint had higher RV end-dyastolic volume indexed (EDVi) [88 (80–109) vs. 81 (70–93) mL/m2, P = 0.0499], lower LVEF and RVEF [51 (37–69) vs. 66 (54–74) %, P = 0.014, and 45 (31–53) vs. 49 (44–55) %, P = 0.010, respectively], and higher ECV [31.5 (26.9–34.3) vs. 27.8 (25.3–30.2) %, P = 0.006]. No significant differences were noted for AV calcium score, LV-EDVi, LV mass indexed, and fibrosis mass indexed (Table 1).
Comparison between patients with high vs. low ECV
The optimal ECV cut-off to distinguish between patients experiencing or not the composite endpoint was 31.3%. Patients with ECV ≥31.3% had higher prevalence of chronic kidney disease [11/29 (38%) vs. 16/84 (19%), P = 0.040], higher TnT [34 (20–41) vs. 20 (15–28) ng/L, P = 0.004], and a tendency towards higher values of BNP [2054 (869–4605) vs. 993 (451–1996) ng/dL, P = 0.053]. Subjects with ECV ≥31.3% had significantly lower LVEF at echocardiography [57 (51–62) % vs. 61 (58–65) %, P = 0.008], while AVAi and AV mean gradient were not significantly different (Table 2).
Table 2.
Clinical data and imaging parameters in patients with ECV higher and lower than 31.3%. Abbreviations as in Table 1
| ECV < 31.3 (n = 84) | ECV ≥ 31.3 (n = 29) | P-value | |
|---|---|---|---|
| Primary endpoint | 10 (12) | 13 (45) | <0.001 |
| Age (years) | 82 (80–85) | 84 (78–86) | 0.464 |
| Male sex (%) | 36 (43) | 18 (62) | 0.074 |
| BMI (kg/m2) | 25 (22.3–27.7) | 25.7 (23.1–27.7) | 0.391 |
| Diabetes (%) | 26 (31) | 10 (35) | 0.725 |
| Hypertension (%) | 64 (76) | 23 (79) | 0.731 |
| Hypercholesterolaemia (%) | 57 (68) | 13 (45) | 0.028 |
| Smoking history (%) | 21 (25) | 8 (28) | 0.783 |
| CKD (%) | 16 (19) | 11 (38) | 0.040 |
| Previous revascularization (%) | 20 (24) | 10 (35) | 0.262 |
| PCI | 13 (16) | 8 (28) | |
| CABG | 6 (7) | 0 (0) | |
| Both | 1 (1) | 2 (7) | |
| LF-LG aortic stenosis | 8 (10) | 7 (24) | 0.046 |
| BNP (ng/dL) (n = 62) | 993 (451–1996) | 2054 (896–4605) | 0.053 |
| TnT (ng/L) (n = 101) | 20 (15–28) | 34 (20–41) | 0.004 |
| Echocardiographic measurements | |||
| LVEF (%) | 61 (58–65) | 57 (51–62) | 0.008 |
| AVA indexed (cm2/m2) | 0.41 (0.35–0.49) | 0.43 (0.37–0.53) | 0.261 |
| AV mean gradient (mmHg) | 46 (40–53) | 41 (35–48) | 0.053 |
| IVS thickness (mm) | 13 (12–14) | 13 (12–15) | 0.510 |
| VPW thickness (mm) | 11 (10–12) | 12 (10–13) | 0.029 |
| Relative wall thickness (mm) | 0.52 (0.55–0.58) | 0.56 (0.46–0.62) | 0.482 |
| CT measurements | |||
| LV-EDV indexed (mL/m2) | 77 (68–91) | 85 (76–113) | 0.013 |
| LVEF (%) | 67 (53–74) | 57 (37–68) | 0.025 |
| LV mass indexed (g/m2) | 83 (70–94) | 94 (80–117) | 0.012 |
| RV-EDV indexed (mL/m2) | 82 (70–94) | 89 (79–104) | 0.053 |
| RV-EF (%) | 48 (44–54) | 46 (38–55) | 0.095 |
| AV calcium score (AU) | 2149 (1461–3387) | 2388 (1515–3102) | 0.783 |
| AVA indexed (mL/m2) | 0.40 (0.35–0.48) | 0.44 (0.36–0.59) | 0.079 |
| CACS (AU) | 759 (227–1593) | 739 (282–2357) | 0.657 |
| ECV (%) | 26.9 (24.8–28.8) | 34.5 (32.5–36.7) | <0.001 |
| Fibrosis mass indexed (g/m2) | 21.9 (18.2–26.8) | 31.7 (26.4–38.1) | <0.001 |
P-values less than 0.05 are depicted in bold.
At CT, patients with ECV ≥31.3% had higher LV-EDVi [85 (76–113) vs. 77 (68–91) mL/m2, P = 0.013] and LV mass indexed [94 (80–117) vs. 83 (70–94) g/m2, P = 0.012], lower LVEF [57 (37–68) vs. 67 (53–74) %, P = 0.025], and higher fibrosis mass indexed [31.7 (26.4–38.1) vs. 21.9 (18.2–26.8) g/m2, P < 0.001]. No significant differences were noted for RV-EF, RV-EDVi, AV calcium score, and AVAi (Table 2).
Correlation between biomarkers of myocardial fibrosis, AS severity, and LV decompensation
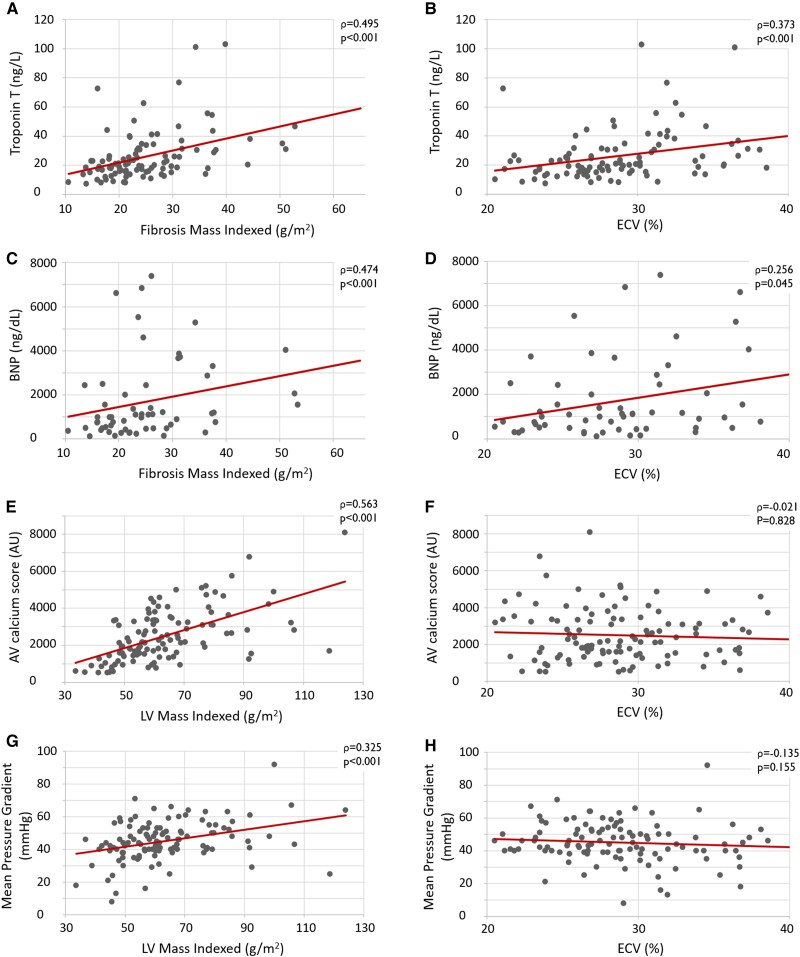
Correlation scatterplots are shown in Figure 3. Among ECV, LV mass indexed, and fibrosis mass indexed, the latter showed the strongest direct correlation with markers of cardiac dysfunction, namely BNP (r = 0.474, P < 0.001), and myocardial damage, namely TnT (r = 0.495, P < 0.001). LV mass indexed had the strongest direct correlation with markers of AS severity, namely AV calcium score (r = 0.563, P < 0.001) and AV mean pressure gradient (r = 0.325, P < 0.001), while ECV was not correlated.
Figure 3.
Correlation scatterplots. Markers of myocardial damage (troponin T) and dysfunction (BNP) showed a stronger direct correlation with the fibrosis mass indexed (A and C, respectively) than with ECV (B and D, respectively). Furthermore, the markers of AS severity, AV calcium score and mean aortic pressure gradient, showed a direct correlation with LV mass indexed (E and G, respectively), while ECV was not correlated (F and H, respectively).
Prognostic value of ECV
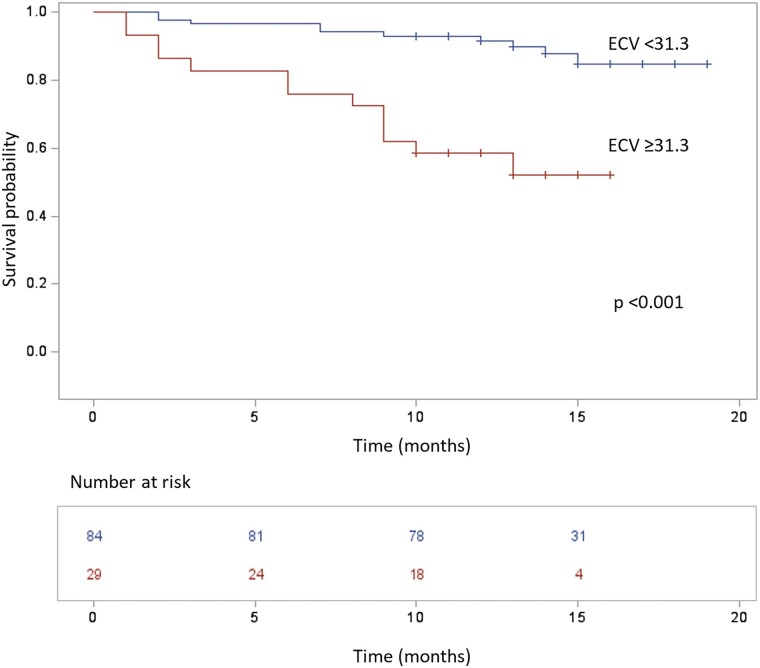
Patients with ECV ≥31.3% showed decreased event-free survival in comparison to patients with ECV <31.3% (log-rank P < 0.001) (Figure 4). At 10 months, the probability of experiencing the composite outcome was 7% for the 84 patients with lower ECV, with death occurring in 6/84 (7%) and HFH in 4/84 (5%), and 41% for the 29 patients with higher ECV, with death occurring in 8/29 (28%) and HFH in 5/29 (17%).
Figure 4.
The Kaplan–Meier survival curve of patients stratified according to ECV. Patients with ECV ≥31.3% (lower line in red) experienced more events of death or HFH than patients with ECV <31.3% (upper line in blue), P < 0.001.
At univariable Cox proportional hazards analysis, CT parameters significantly associated with increased risk of death or HFH were LV- and RV-EDVi, LV- and RV-EF, AVAi, ECV, and fibrosis mass indexed, along with US-derived LVEF and mean pressure gradient (Table 3). At multivariable Cox proportional hazards analysis, significant predictors of death or HFH were US-derived AVAi, aortic mean pressure gradient, and ECV (Table 3). Therefore, ECV was the only CT parameter predictive of outcome. Patients with ECV values ≥31.3% had a 5.92-fold increased risk of adverse outcome in comparison to patients with ECV <31.3% (HR = 5.92, 95% CI 2.37–14.75).
Table 3.
Univariate (upper) and multivariable (lower) Cox regression analysis. Abbreviations as in Table 1
| Univariate Cox regression analysis | |||
|---|---|---|---|
| HR | 95% CI | P-value | |
| Age (years) | 1.05 | 0.97–1.14 | 0.254 |
| Male sex (%) | 1.03 | 0.45–2.34 | 0.945 |
| BMI (kg/m2) | 0.95 | 0.86–1.05 | 0.322 |
| CKD (%) | 1.14 | 0.45–2.34 | 0.777 |
| BNP (ng/dL) | 1.00 | 1.00–1.00 | 0.592 |
| TnT (ng/L) | 1.00 | 0.99–1.01 | 0.539 |
| Echocardiographic measurements | |||
| LVEF (%) | 0.95 | 0.92–0.98 | 0.001 |
| AVA indexed (cm2/m2) (unit = 0.1) | 0.88 | 0.59–1.31 | 0.530 |
| AV mean gradient (mmHg) | 0.95 | 0.92–0.98 | <0.001 |
| CT measurements | |||
| LV-EDV indexed (mL/m2) | 1.02 | 1.00–1.04 | 0.019 |
| LVEF (%) | 0.97 | 0.95–0.99 | 0.005 |
| LV mass indexed (g/m2) | 1.01 | 0.99–1.02 | 0.347 |
| RV-EDV indexed (mL/m2) | 1.02 | 1.01–1.04 | 0.003 |
| RV-EF (%) | 0.95 | 0.91–0.98 | 0.002 |
| AV calcium score (AU) | 1.00 | 1.00–1.00 | 0.069 |
| AVA indexed (cm2/m2) | 1.39 | 1.00–1.92 | 0.049 |
| ECV (%) | 1.12 | 1.03–1.22 | 0.007 |
| ECV ≥31.3% | 5.11 | 2.22–11.75 | <0.001 |
| Fibrosis mass indexed (g/m2) | 1.04 | 1.00–1.08 | 0.047 |
| Multivariable Cox regression analysis | |||
|---|---|---|---|
| HR | 95% CI | P-value | |
| Echocardiographic measurements | |||
| AVA indexed (cm2/m2) (unit = 0.1) | 0.55 | 0.35–0.88 | 0.013 |
| AV mean gradient (mmHg) | 0.95 | 0.92–0.98 | <0.001 |
| CT measurements | |||
| ECV ≥31.3% | 5.92 | 2.37–14.75 | <0.001 |
P-values less than 0.05 are depicted in bold.
Interobserver agreement
The interobserver agreement for ECV quantification was excellent [intraclass correlation coefficient: 0.94 (95% CI 0.92–0.96), P < 0.001].
Discussion
The main result of our study is that, in consecutive prospectively enroled patients with severe AS, ECV measured at CT exam for TAVI planning significantly predicts long-term clinical outcome.
Multiple studies have shown that, in patients with severe AS, myocardial fibrosis is an important determinant of progressive LV dysfunction and clinical adverse outcome, including end-stage heart failure and death.10,21,22 The main mechanism leading to LV dysfunction is progressive myocardial hypertrophy and fibrosis deposition determined by the increased afterload due to the stenotic AV.2 A recent study combining CMR and histology has shown that myocardial fibrosis can develop in complex patterns including endocardial fibrosis, subendocardial microscars, and diffuse interstitial fibrosis.23 CMR studies have shown that scarring fibrosis has an important prognostic value in the AS setting.8,22,24 However, also diffuse interstitial fibrosis has a prognostic significance. In a multicentre CMR study, Everett et al.10 demonstrated that, in patients with severe AS candidate to AV replacement, increased ECV predicted all-cause mortality at long-term follow-up. However, performing CMR in the routine assessment of TAVI candidates may be highly impractical due to limited patient compliance, costs, and scanner availability constrains. Thus, the possibility of evaluating ECV using the CT examination already recommended for TAVI planning19 is particularly appealing.
Few studies have been conducted to assess whether ECV measured with CT can predict long-term prognosis in AS patients undergoing TAVI.
In a retrospective study from Tamarappoo et al.17 enroling 150 patients affected by low-flow low-gradient AS, ECV was associated with increased risk of heart failure hospitalization or death after TAVI.
Suzuki et al.25 and Hammer et al.26 have found association between ECV and long-term mortality in two retrospective analyses on 95 and 75 AS patients; however, both studies included mixed population undergoing either TAVI or surgical AV replacement.
Our prospective study demonstrates that ECV derived from CT planning exam is an independent predictor of prognosis after TAVI. The robustness of the study results derives from its prospective design and relatively large sample size. Its high clinical value descends from the enroling of a population including only TAVI recipients. Moreover, differently from the retrospective analysis of Tamarappoo et al.,17 our study has demonstrated the prognostic value of ECV in an unselected population of TAVI candidates with either high- or low-gradient AS, thus representing the real-world clinical practice.
Our results highlight that ECV is an independent prognostic predictor in TAVI candidates that allows to explore another domain inaccessible to the standard pre-TAVI work-up, obtainable by simple addition of a low-dose scan to the standard CT for TAVI planning.
Our results are in agreement with previous reports showing that a decreased AVA,27 marker of stenosis severity, and a decreased AV pressure gradient,28 marker of LV dysfunction, carry a dismal prognosis.
Thus, the measurement of ECV could become pivotal for better risk stratification and treatment tailoring to avoid futile TAVI procedures and consequent optimization of patient management and of healthcare resources.
As previously reported by CMR studies, ECV was correlated to laboratory markers of myocardial damage and decompensation (TnT and pro-BNP).23 The correlation was stronger for the LV fibrosis mass, a parameter that accounts also for myocardial mass, suggesting that myocardial damage may be proportional to the degree of LV adverse remodelling.
However, ECV was not correlated to haemodynamic parameters of AS severity, in agreement with previous CMR10,23 and CT25 studies, suggesting that higher ECV, being a marker of increased interstitial fibrosis, may be associated to LV dysfunction and consequent decreased AV mean pressure gradient.
Moreover, we observed that ECV was also not correlated to the most robust CT parameter of AS severity, namely AV calcium score.
These findings may suggest that high ECV, instead of being the result of interstitial fibrosis deposition solely due to long-standing AS, may also be caused by other conditions, such as inflammatory or ischaemic cardiomyopathies,29 and amyloidosis.
The latter is highly relevant since it is more prevalent among older patients. In fact, previous studies have shown that it may affect between 6 and 16% of TAVI candidates,30–32 causing a significant increase in ECV.31 Thus, a proportion of our patients with higher ECV might have had severe AS and concurrent amyloidosis, partially explaining their worse prognosis.
Study limitations
Our study has some limitations. First, we enroled a limited sample size, although this is the largest prospective cohort of severe AS patients in which the prognostic value of ECV on long-term TAVI efficacy was assessed, according to our best knowledge. Second, given that we did not perform CMR in this cohort, the standard of reference for non-invasive assessment of myocardial fibrosis was not available. Finally, scintigraphy was not performed; thus, some patients with elevated ECV may have had concomitant amyloidosis, with potential confounding effects on the outcome.
Conclusion
In patients with severe AS undergoing TAVI, myocardial ECV measured at CT performed for TAVI planning predicts unfavourable outcome at long-term follow-up. ECV measurement may be routinely integrated in the TAVI planning CT examination and suggested in multiparametric scores to improve patients’ risk stratification and management.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Supplementary Material
Contributor Information
Davide Vignale, Clinical and Experimental Radiology Unit, Experimental Imaging Center, IRCCS San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy; School of Medicine, Vita-Salute San Raffaele University, via Olgettina 58, Milan 20132, Italy.
Anna Palmisano, Clinical and Experimental Radiology Unit, Experimental Imaging Center, IRCCS San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy; School of Medicine, Vita-Salute San Raffaele University, via Olgettina 58, Milan 20132, Italy.
Chiara Gnasso, Clinical and Experimental Radiology Unit, Experimental Imaging Center, IRCCS San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy; School of Medicine, Vita-Salute San Raffaele University, via Olgettina 58, Milan 20132, Italy.
Davide Margonato, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy.
Davide Romagnolo, School of Medicine, Vita-Salute San Raffaele University, via Olgettina 58, Milan 20132, Italy; Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy.
Simone Barbieri, Experimental Imaging Center, IRCCS San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy.
Giacomo Ingallina, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy.
Stefano Stella, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy.
Marco Bruno Ancona, Interventional Cardiology Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy.
Matteo Montorfano, Interventional Cardiology Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy.
Francesco Maisano, School of Medicine, Vita-Salute San Raffaele University, via Olgettina 58, Milan 20132, Italy; Department of Cardiac Surgery, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy.
Eustachio Agricola, School of Medicine, Vita-Salute San Raffaele University, via Olgettina 58, Milan 20132, Italy; Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy.
Antonio Esposito, Clinical and Experimental Radiology Unit, Experimental Imaging Center, IRCCS San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy; School of Medicine, Vita-Salute San Raffaele University, via Olgettina 58, Milan 20132, Italy.
Funding
None declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Osnabrugge RLJ, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, Lereun CMet al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013;62:1002–12. [DOI] [PubMed] [Google Scholar]
- 2. Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol 2012;60:1854–63. [DOI] [PubMed] [Google Scholar]
- 3. Bing R, Cavalcante JL, Everett RJ, Clavel MA, Newby DE, Dweck MR. Imaging and impact of myocardial fibrosis in aortic stenosis. JACC: Cardiovascular Imaging 2019;12:283–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coffey S, Cox B, Williams MJA. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol 2014;63:2852–61. [DOI] [PubMed] [Google Scholar]
- 5. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LGet al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607. [DOI] [PubMed] [Google Scholar]
- 6. Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, Zajarias Aet al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial (Cohort A). J Am Coll Cardiol 2012;60:548–58. [DOI] [PubMed] [Google Scholar]
- 7. Chen H, Zeng J, Liu D, Yang Q. Prognostic value of late gadolinium enhancement on CMR in patients with severe aortic valve disease: a systematic review and meta-analysis. Clin Radiol 2018;73:983.e7–983.e14. [DOI] [PubMed] [Google Scholar]
- 8. Barone-Rochette G, Piérard S, Meester De Ravenstein CD, Seldrum S, Melchior J, Maes Fet al. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J Am Coll Cardiol 2014;64:144–54. [DOI] [PubMed] [Google Scholar]
- 9. Musa TA, Treibel TA, Vassiliou VS, Captur G, Singh A, Chin Cet al. Myocardial scar and mortality in severe aortic stenosis: data from the BSCMR Valve Consortium. Circulation 2018;138:1935–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Everett RJ, Treibel TA, Fukui M, Lee H, Rigolli M, Singh Aet al. Extracellular myocardial volume in patients with aortic stenosis. J Am Coll Cardiol 2020;75:304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee H, Park JB, Yoon YE, Park EA, Kim HK, Lee Wet al. Noncontrast myocardial T1 mapping by cardiac magnetic resonance predicts outcome in patients with aortic stenosis. JACC Cardiovasc Imaging 2018;11:974–83. [DOI] [PubMed] [Google Scholar]
- 12. Palmisano A, Vignale D, Peretto G, Busnardo E, Calcagno C, Campochiaro Cet al. Hybrid FDG-PET/MR or FDG-PET/CT to detect disease activity in patients with persisting arrhythmias after myocarditis. JACC Cardiovasc Imaging 2021;14:288–92. [DOI] [PubMed] [Google Scholar]
- 13. Palmisano A, Vignale D, Benedetti G, Del Maschio A, De Cobelli F, Esposito A. Late iodine enhancement cardiac computed tomography for detection of myocardial scars: impact of experience in the clinical practice. Radiol Medica 2020;125:128–36. [DOI] [PubMed] [Google Scholar]
- 14. Palmisano A, Vignale D, Tadic M, Moroni F, De Stefano D, Gatti Met al. Myocardial late contrast enhancement CT in troponinpositive acute chest pain syndrome. Radiology 2022;302:545–53. [DOI] [PubMed] [Google Scholar]
- 15. Esposito A, Palmisano A, Barbera M, Vignale D, Benedetti G, Spoladore Ret al. Cardiac computed tomography in troponin-positive chest pain: sometimes the answer lies in the late iodine enhancement or extracellular volume fraction map. JACC Cardiovasc Imaging 2019;12:745–8. [DOI] [PubMed] [Google Scholar]
- 16. Esposito A, Palmisano A, Antunes S, Colantoni C, Rancoita PMV, Vignale Det al. Assessment of remote myocardium heterogeneity in patients with ventricular tachycardia using texture analysis of late iodine enhancement (LIE) cardiac computed tomography (cCT) images. Mol Imaging Biol 2018;20:816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamarappoo B, Han D, Tyler J, Chakravarty T, Otaki Y, Miller Ret al. Prognostic value of computed tomography–derived extracellular volume in TAVR patients with low-flow low-gradient aortic stenosis. JACC Cardiovasc Imaging 2020;13:2591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han D, Tamarappoo B, Klein E, Tyler J, Chakravarty T, Otaki Yet al. Computed tomography angiography-derived extracellular volume fraction predicts early recovery of left ventricular systolic function after transcatheter aortic valve replacement. Eur Heart J Cardiovasc Imaging 2021;22:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Stephan B, Bauersachs Jet al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561–632. [DOI] [PubMed] [Google Scholar]
- 20. Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein Set al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 2017;18:254–75. [DOI] [PubMed] [Google Scholar]
- 21. Weidemann F, Herrmann S, Störk S, Niemann M, Frantz S, Lange Vet al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009;120:577–84. [DOI] [PubMed] [Google Scholar]
- 22. Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio ROet al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol 2010;56:278–87. [DOI] [PubMed] [Google Scholar]
- 23. Treibel TA, López B, González A, Menacho K, Schofield RS, Ravassa Set al. Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non-invasive study in 133 patients. Eur Heart J 2018;39:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herrmann S, Fries B, Salinger T, Liu D, Hu K, Gensler Det al. Myocardial fibrosis predicts 10-year survival in patients undergoing aortic valve replacement. Circ Cardiovasc Imaging 2018;11:e007131. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki M, Toba T, Izawa Y, Fujita H, Miwa K, Takahashi Y, et al. Prognostic impact of myocardial extracellular volume fraction assessment using dual-energy computed tomography in patients treated with aortic valve replacement for severe aortic stenosis. J Am Heart Assoc 2021;10:e020655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hammer Y, Barkan YT, Abelow A, Orvin K, Aviv Y, Bar N, et al. Myocardial extracellular volume quantification by computed tomography predicts outcomes in patients with severe aortic stenosis. PLoS One 2021;16:e0248306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gamaza Chulián S, Díaz Retamino E, Carmona García R, Serrano Muñoz B, León Jiménez J, González Estriégana Set al. Prognostic value of aortic valve area normalized to body size in native aortic stenosis. Rev Española Cardiol (English Ed) 2021;74:44–50. [DOI] [PubMed] [Google Scholar]
- 28. Conrotto F, D’Ascenzo F, D’Amico M, Moretti C, Pavani M, Scacciatella Pet al. Outcomes of patients with low-pressure aortic gradient undergoing transcatheter aortic valve implantation: a meta-analysis. Catheter Cardiovasc Interv 2017;89:1100–6. [DOI] [PubMed] [Google Scholar]
- 29. Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2 and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imagin. J Cardiovasc Magn Reson 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castano A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca Aet al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J 2017;38:2879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scully PR, Patel KP, Saberwal B, Klotz E, Augusto JB, Thornton GDet al. Identifying cardiac amyloid in aortic stenosis: ECV quantification by CT in TAVR patients. JACC Cardiovasc Imaging 2020;13:2177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Treibel TA, Fontana M, Gilbertson JA, Castelletti S, White SK, Scully PR, et al. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging 2016;9:e005066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.