Summary:
Lipedema is a chronic and progressive disease that may compromise lymphatic function. Although suction-assisted lipectomy (SAL) is considered a safe treatment for lipedema patients, the lymphatic repercussions of this surgical procedure are not fully understood. There is not enough evidence to support the role of SAL in lymphatic function treatment in lipedema. Here, we report a case of lymphatic drainage improvement after lipedema treatment with SAL. Tumescent SAL was performed in the deep subcutaneous layer, preserving the superficial and muscular lymphatic vessels. Pre- and postsurgical lymphoscintigraphy was equally documented under the Genoa protocol. A 34-year-old female patient presented with painful enlargement of the arms and lower limbs caused by lipedema. The patient had undergone conservative treatment with mild improvement in pain and heaviness. Lymphoscintigraphy showed slowed radiotracer progression on the left lower limb, collateral and tortuous lymphatic vessels on the right lower limb, and exuberant radiopharmaceutical concentration on the inguinal chain. Nine months after SAL was performed, the patient underwent another lymphoscintigraphy, which exhibited normalized radiopharmaceutical progression time and normal and symmetrical lymphatic vessel patterns. Collateral lymphatic paths and tortuosity vessels were no longer identified. Furthermore, the patient reported significant improvement in pain and the limb’s appearance. Tumescent SAL is not only efficient and safe in treating lipedema, but may also be responsible for improvement in lymphatic drainage in lipedema patients. Additional prospective studies are fundamental to reinforce the current evidence and possibly yield predicting information about the tumescent liposuction eligibility in the improvement of lymphatic drainage.
Lipedema is an abnormal, symmetrical, subcutaneous adipose tissue deposition involving inflammation and fibrosis in the lower extremities and, less often, the trunk and upper extremities.1,2 The world prevalence is nearly 11% among adult women, and diagnosis is mainly based on clinical features.1
Since there is progressive tissue modification, such as increased cutaneous thickness, fibrotic nodules, and lymphatic impairment, early diagnosis and treatment are imperative.2 The main goal of the treatment is to interrupt fibrosis progression and improve quality of life.
Lipedema management includes psychological counseling, diet, physical exercising, decongestive lymphatic therapy, compression garments, and surgery.1,3,4 Although strong evidence supports tumescent liposuction as an effective treatment, which promotes symptom relief and functional and aesthetic improvement, there is only an incipient understanding of surgery’s effect on the lymphatic vessels.3–5
CASE DESCRIPTION
Informed consent was obtained from the patient, and the study was approved by Instituto D’Or Pesquisa e Ensino Research Ethics Committee and the Biomedical Science Institute of the University of Sao Paulo’s ethics committee.
A 34-year-old woman presented with chronic bilateral enlargement of the arms and lower extremities, present since puberty and gradually progressing over time. The patient reported discomfort, lower limb heaviness, and frequent, spontaneous bruises. She had undergone dietary restrictions, lymphatic drainages, regular physical exercises, and weight loss attempts with mild improvement.
The patient presented with obesity (96.3 kg, 1.63 m, 36.24 kg/m2) and significant enlargement of the arms and lower extremities, especially the thighs and knees. The forearms, hands, and feet were not affected. There was no clinical evidence of edema in the physical examination and bilateral negative Stemmer’s sign.
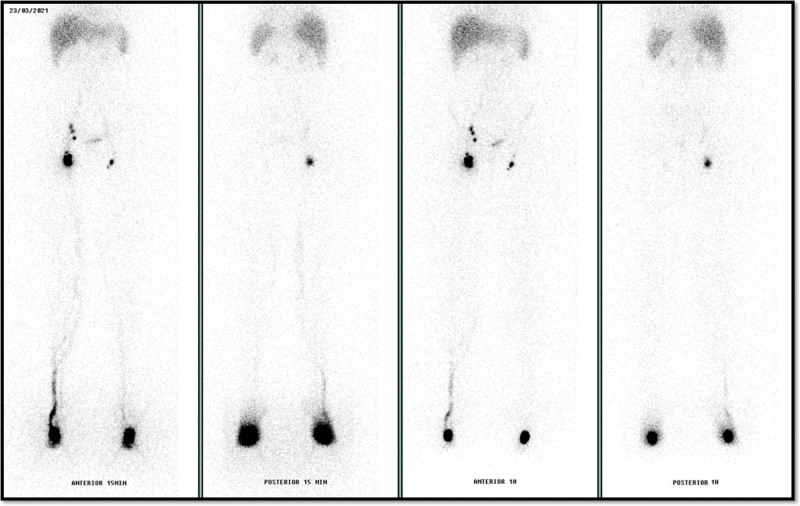
Lymphoscintigraphy6 evidenced slowed radiotracer progression on the left lower limb and normal drainage speed on the right limb. Collateral and tortuous lymphatic vessels were identified on the right lower limb and also an exuberant concentration of 99 mTc-phytate on the inguinal chain. Right external iliac lymph nodes were visualized in early images (Fig. 1).
Fig. 1.
Anterior and posterior images of lymphoscintigraphy with 99 mTc-phytate showing radiotracer progression slightly slowed on the left lower limb, collateral and tortuous lymphatic vessels on the right limb, and exuberant concentration on the inguinal chain. These images were achieved using a GE gamma camera, Discovery NM630. Anterior and posterior projection images of the lower limbs were acquired 15 and 60 minutes after subcutaneous injection of 37 MBq (1 mCi) of 99mTc-phytate applied in the dorsal region of the feet. The gamma camera had a large field of view and was equipped with a parallel hole low-energy high-resolution collimator (±1% window centered on the 140-keV energy peak of 99 mTc).
After preoperative evaluation, the patient underwent tumescent suction-assisted lipectomy (SAL) of the arms and lower extremities. The level of liposuction was the deep subcutaneous layer to preserve the superficial and muscular lymphatic vessels and to avoid superficial irregularities. Fat tissue aspirate (3900 mL) was removed from each lower limb with minimal bleeding (40 mL of blood sedimented in the collector). Subdermal LASER diode of 980 nm was applied after liposuction to promote skin tightening in the thighs. In addition, bilateral arm lift was performed to treat skin laxity. Low molecular weight heparin was given for deep venous thrombosis prophylaxis.
Postoperative pain intensity was rated 7 according to the numeric rating scale (NRS-11) until the fourth day after surgery. Subsequently, the patient’s discomfort was rated 3 and gradually faded until the third week after surgery. The given discomfort and edema improved with analgesics (dipyrone, profenid, and oxycodone) and lymphatic drainage.
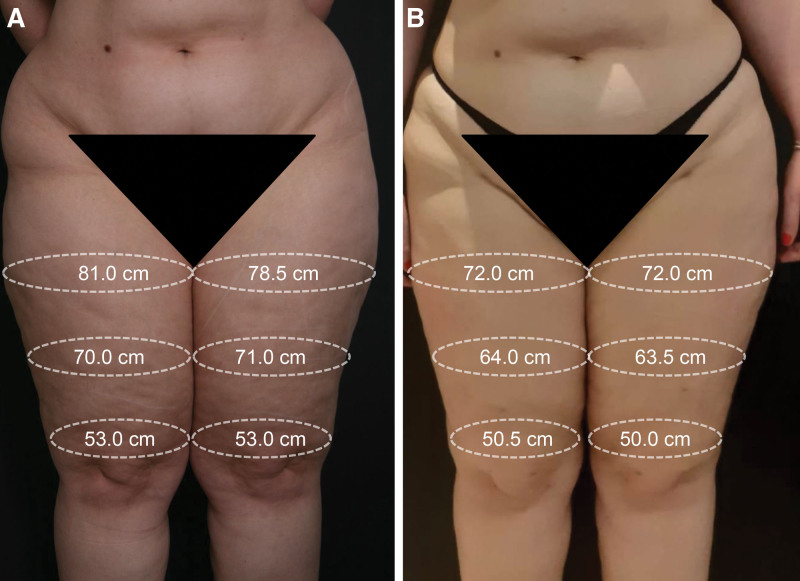
Compression garments and bandages were applied every 24 hours in the first month after surgery. Afterward, the patient wore compression garments for half of the day until the third postoperative month and intermittently until the sixth postoperative month, and after that period, she stopped using compression garments. The patient reported symptom improvement (pain, discomfort, and leg heaviness), and satisfaction with the limbs’ appearance (Fig. 2).
Fig. 2.
Comparative images of the patient’s lower limbs. A, Before surgery. B, After surgery. The bilateral lower limb liposuction focused on thighs and knees, where the most severe discomfort and extensive enlargement were observed. The later pictures and measurements were obtained nine months after surgery.
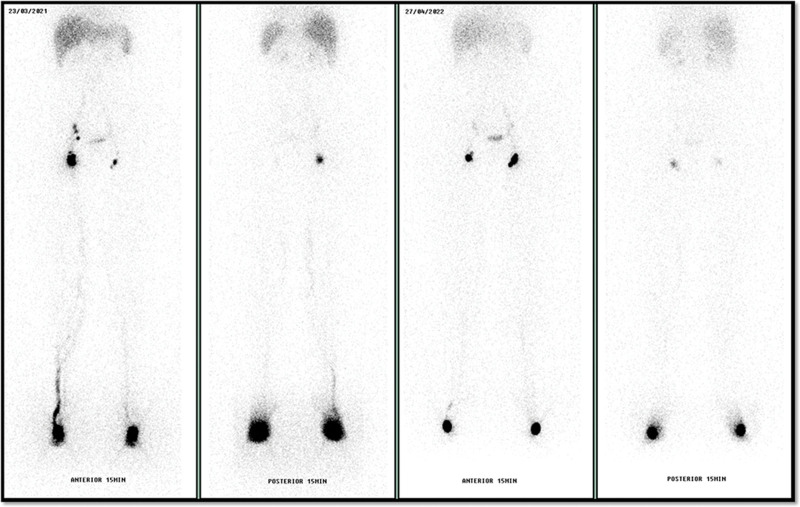
A second lymphoscintigraphy (under identical protocol6) was performed 9 months after surgery with resolution of the lymphatic abnormalities, normal radiopharmaceutical progression time, and symmetrical drainage pattern. Collateral lymphatic paths and tortuous vessels were no longer identified (Fig. 3).
Fig. 3.
Comparative images of pre and postoperative lymphoscintigraphy with 99 mTc-phytate showing resolution of the lymphatic abnormalities.
DISCUSSION
Lipedema patients usually present tortuous and convoluted lymphatic vessels (75%), with normal progression time to inguinal lymph nodes (>90%), and may present dermal backflow in early lymphatic failure.2 Patients may present slowed lymphatic drainage, which can be asymmetrical, despite the symmetrical nature of the disease.7
Despite the concern of possible lymphatic damage due to surgical trauma, only one case series was found that described lymphatic injuries in three lipedema patients after SAL.8 Strong evidence supports that, when performed by an experienced team, tumescent liposuction can improve quality of life with lymphatic safety.3,4,9
Notwithstanding, paucity of data suggests postsurgical lymphatic improvement. In 2019, Van de Pas et al9 found mild lymphatic improvement in inguinal uptake after surgery. However, to the authors’ knowledge, no other work describes improvement in the progression pattern and resolution of collateral lymphatic paths and tortuous vessels after tumescent liposuction.
Although the mechanism of amelioration of lymphatic function after tumescent SAL is not completely elucidated, in a chronic inflammatory environment, INF-γ and TGF-β1, antilymphangiogenic cytokines are elevated. INF-γ is produced by T-cells and functions as a potent inhibitor of lymphatic proliferation and differentiation. Lymphatic regeneration is impaired by the increased expression of IFN-γ and TGF-β1 that occurs in T-cell inflammation.10,11
Obesity is also correlated with lymphatic dysfunction, with mouse and rat models showing leaky and impaired collecting vessel contraction. Elevated INF-γ and TGF-β1 were observed in obese mice and humans.11 Therefore, resolving T-cell inflammation and reduction of the expression of INF-γ and TGF-β1 may be the key role of SAL in lymphatic outcomes.
The setting of obesity can lead to injury, inflammation, and further lymphatic blockage from fat overgrowth. In this scenario, the lymphatic system subsequently becomes overloaded and cannot accommodate flow.1 In addition, the excess of lymphatic fluid can upregulate adipose differentiation and generation.1
Hence, by eliminating excessive lymph, liposuction may decrease adipogenesis. Furthermore, by decreasing the pressure of fat cells on lymph collectors, SAL could improve lymphatic function and diminish previous lymphatic and circulatory disturbances. SAL may also reduce the cellular and metabolic load on the lymphatic system by bypassing its native routes and increasing the local drainage.1
Therefore, the authors hypothesize that lymphatic function response to surgery relies on the manipulation of the lymphangiogenesis/antilymphangiogenesis axis, as illustrated in Supplemental Digital Content 1. (See figure, Supplemental Digital Content 1, which displays the factors that interfere in the lymphatic response to modified SAL. Lymphangiogenesis is stimulated in response to a decrease in chronic inflammation. Prolymphangiogenic factors, VEGF-C, VEGF-D, and VEGF-A, are secreted by macrophages following chemoattractants expressed by lymphatic endothelial cells. Otherwise, INF-γ and TGF-β1, antilymphangiogenic cytokines, are inhibitors of lymphatic proliferation and differentiation that are expressed in subcutaneous tissue inflammation, http://links.lww.com/PRSGO/C633.)11
CONCLUSIONS
Tumescent SAL may improve lymphatic function in lipedema patients. Understanding the factors that influence lymphatic behavior after SAL may lead to safer treatments for lipedema patients. Additional prospective studies are fundamental to reinforce the current evidence and possibly yield predicting information about tumescent liposuction eligibility in the improvement of lymphatic drainage.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online 21 June 2023.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Gould DJ, El-Sabawi B, Goel P, et al. Uncovering lymphatic transport abnormalities in patients with primary lipedema. J Reconstr Microsurg. 2020;36:136–141. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen JC, Aldrich MB, Fife CE, et al. Lymphatic function and anatomy in early stages of lipedema. Obesity. 2022;30:1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandhofer M, Hanke CW, Habbema L, et al. Prevention of progression of lipedema with liposuction using tumescent local anesthesia: results of an international consensus conference. Dermatol Surg. 2020;46:220–228. [DOI] [PubMed] [Google Scholar]
- 4.Aksoy H, Karadag AS, Wollina U. Cause and management of lipedema-associated pain. Dermatol Ther. 2021;34:e14364. [DOI] [PubMed] [Google Scholar]
- 5.van de Pas CB, Boonen RSM, Stevens S, et al. Does tumescent liposuction damage the lymph vessels in lipoedema patients? Phlebology. 2020;35:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villa G, Campisi CC, Ryan M, et al. Procedural recommendations for lymphoscintigraphy in the diagnosis of peripheral lymphedema: the genoa protocol. Nucl Med Mol Imaging. 2019;53:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilancini S, Lucchi M, Tucci S, et al. Functional lymphatic alterations in patients suffering from lipedema. Angiology. 1995;46:333–339. [DOI] [PubMed] [Google Scholar]
- 8.Wright TF, Herbst KL. A case series of lymphatic injuries after suction lipectomy in women with lipedema. Am J Case Rep. 2022;23:e935016–e935011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Pas CB, Boonen RSM, Stevens S, et al. Does tumescent liposuction damage the lymph vessels in lipoedema patients? Phlebology. 2020;35:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Nicolls MR, Tian W, et al. Lymphatic dysfunction, leukotrienes, and lymphedema. Annu Rev Physiol. 2018;80:49–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norden PR, Kume T. The role of lymphatic vascular function in metabolic disorders. Front Physiol. 2020;11:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.