Fig. 1.
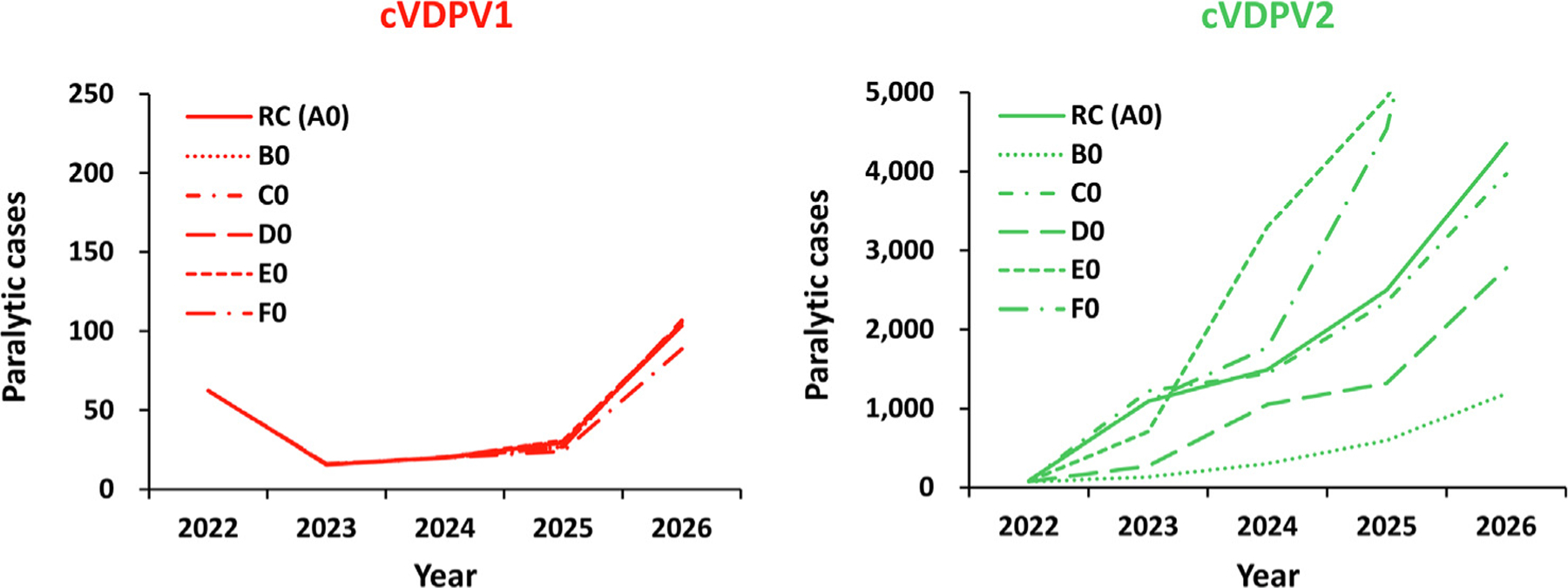
Expected global number of polio cases by year for 100 stochastic iterations of the different vaccine characteristics scenarios for 2022–2026 compared to the reference case (see main text for descriptions). Notes: All scenarios use the characteristics of the reference case (Table 1 bottom, row 0). Abbreviations: cVDPV(1,2), circulating vaccine-derived poliovirus (type 1 or 2); RC (A0) indicates the reference case, which uses monovalent Sabin OPV2 (A); B0 indicates the use of novel OPV2 (nOPV2) assuming the same effectiveness, no reversion, and no vaccine-associated paralytic polio (VAPP) [16]; C0 indicates the use of nOPV2 assuming the same effectiveness and same VAPP as mOPV2, but reduced reversion relative to mOPV2; D0 assumes nOPV2 with reduced effectiveness, no reversion, and no VAPP; E0 assumes nOPV2 with reduced effectiveness and VAPP and less reversion relative to mOPV2; and F0 uses mOPV2 until April 30, 2024 and then inactivated poliovirus vaccine (IPV) for the rest of the time horizon.
