Fig. 2.
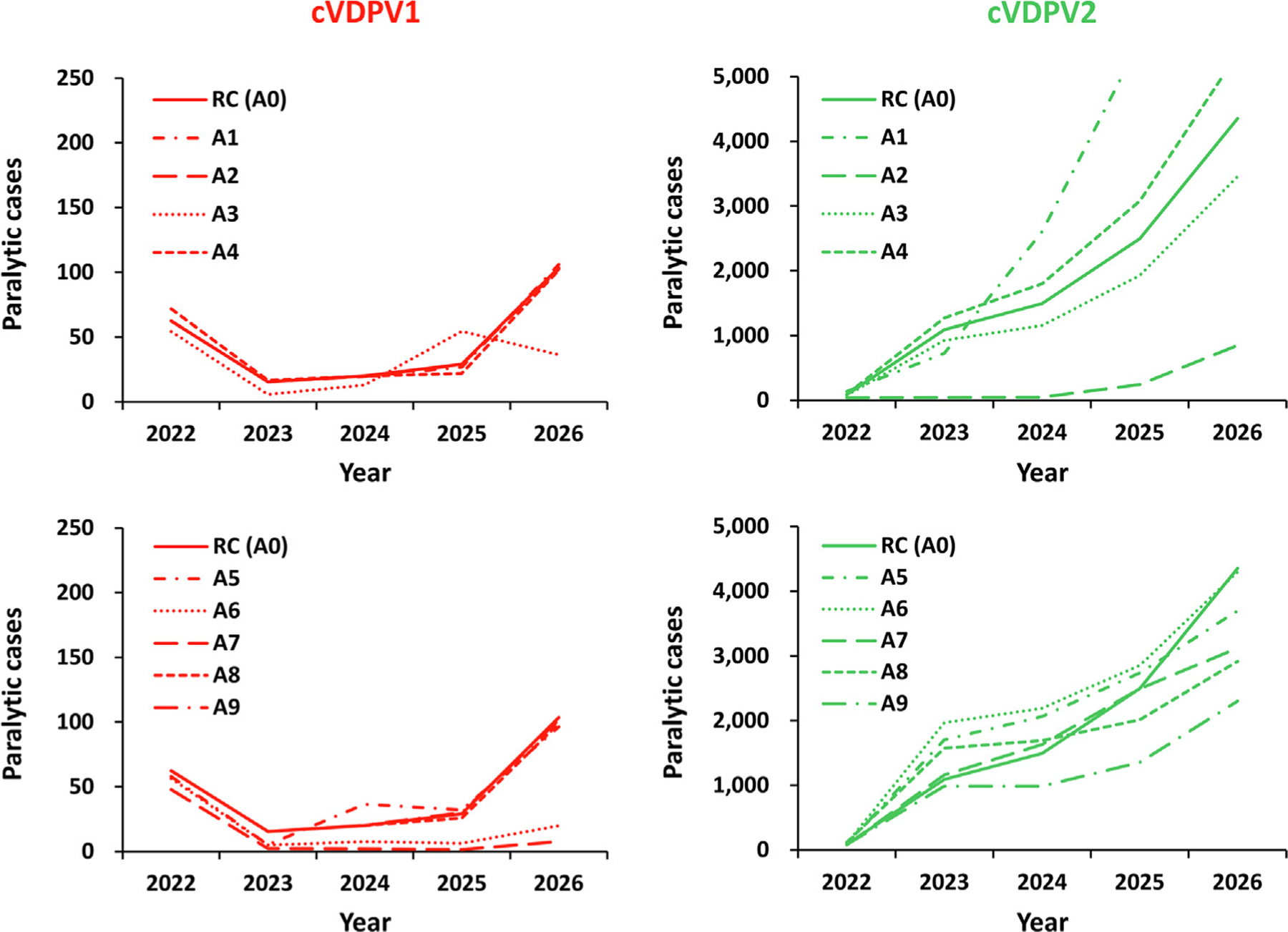
Expected global number of polio cases by year for 100 stochastic iterations of univariate outbreak response SIA change scenarios using mOPV2 for direct comparison to the reference case for 2022–2026 (see main text for descriptions). Notes: All scenarios use monovalent Sabin OPV2 (Table 1, top, row A) and all or all but one of the reference case characteristics (Table 1, bottom, row 0). Abbreviations: cVDPV(1,2), circulating vaccine-derived poliovirus (type 1 or 2); RC (A0) indicates the reference case, which assumes response in the outbreak subpopulation and 4 subpopulations in the same block at highest-risk (1 + 4) in blocks with high transmissibility that starts 45 days after case detection with 2 rounds with immunization intensity consistent with recent experience in preventive campaigns, and target children < 5 years of age; A1 reduces the scope of the response to just the outbreak subpopulation (1 + 0) in blocks with high transmissibility; A2 increase the scope to all 10 subpopulations in the block (1 + 9) in blocks with high transmissibility; A3 assumes a shorter delay of 30 days to the first round; A4 assumes a longer delay of 60 days to the first round; A5 assumes three rounds; A6 assumes four rounds; A7 assumes high intensity rounds (i.e., 80% immunization coverage, 70% repeated miss probability) for all response rounds; A8 assumes an expanded target age group of children < 10 years of age for all responses; and A9 assumes an expanded target age group of children < 10 years of age for outbreaks in subpopulations with no OPV2 use since 2016.
