Abstract
Background
Hemophilus influenzae is a gram-negative coccobacillus. Non-typeable H. influenzae infection is a significant cause of disease that activates the inflammatory pathway involving the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome. A gain-of-function mutation in NLRP3 results in cryopyrin-associated periodic syndromes characterized by inflammatory conditions in the lungs, skin, joints, and eyes but not in the gut. This leads to homeostasis of the gut microbiota, which reduces inflammation and may have protective effect against colorectal cancer (CRC). This study aimed to evaluate the correlation between H. influenzae infection and the incidence of CRC.
Methods
A retrospective study was conducted from 2010 to 2019 using a HIPAA-compliant national database. ICD-10, ICD-9, CPT, and National Drug Codes were used to identify patients with or without a history of H. influenzae infection. Standard statistical methods were used to analyze the outcomes.
Results
The query was analyzed and matched, resulting in 13,610 patients in both groups. The incidence of CRC was 167 and 446 in the H. influenzae and control groups, respectively. The difference was statistically significant with P < 2.2 ×10-16 and an odds ratio of 0.41 (95% confidence interval: 0.36 - 0.47). Additionally, the groups were further evaluated and matched by treatment, which resulted in a statistically significant decrease in CRC incidence in the H. influenzae group.
Conclusion
This study showed a statistically significant correlation between H. influenzae and the reduced incidence of CRC. This reduction in CRC in patients with a history of H. influenzae infection suggests a potential link to the NLRP3 inflammasome, which should be further studied.
Keywords: Colorectal cancer, H. influenzae, Non-typeable H. influenzae, NLRP3 inflammasome, Microbiome, Gut-lung axis
Introduction
Colorectal cancer (CRC) is the third most common cancer and the fourth most lethal cancer, killing almost 700,000 people every year [1]. CRC can develop sporadically in the setting of hereditary cancer syndromes or inflammatory bowel diseases. Microbes have been associated with the progression and initiation of inflammation and inflammatory bowel disease (IBD) [1, 2]. IBD includes ulcerative colitis and Crohn’s disease, which, alongside old age, hereditary syndromes, adenomas, smoking, alcohol, obesity, diet high in processed meat or high-fat and low fiber, are risk factors for CRC [3, 4]. Accumulating evidence suggests that the human microbiota contributes to the development of CRC. Colonic bacteria and their metabolites are thought to contribute to colorectal carcinogenesis via DNA damage and production of pro-inflammatory environments, while metabolites produced by other bacteria may exert a protective function against damage to the intestinal wall [3, 4]. Homeostasis of the microbiota may therefore be a significant factor determining cancer risk [4, 5].
Hemophilus influenzae (H. influenzae) is a gram-negative coccobacillus that has recently been associated with the microbiome. Its various clinical manifestations include meningitis, epiglottitis, cellulitis, pneumonia, and septic arthritis [6]. However, due to the widespread childhood immunization program in the 1990s, the incidence of typeable H. influenzae type b (Hib) infection was reduced. Currently, a significant cause of invasive H. influenzae infection is the non-typeable Hemophilus influenzae (NTHi) [6, 7]. Recent investigations of NTHi suggest activation of the inflammatory pathway involving upregulation of the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome [7].
While inflammation is a protective immune response to harmful stimuli including pathogens or irritants, excessive inflammation can lead to persistent infection or chronic inflammatory diseases [8]. Inflammasomes are immune system receptors/sensors consisting of a cytoplasmic multimeric protein complex that regulates the activation of cysteine protease caspase-1 and are pivotal to drive inflammation [8, 9]. Upon activation, the NLRP3 inflammasome converts pro-caspase-1 to active caspase-1, which forms mature interleukin (IL)-1β and IL-18 pro-inflammatory cytokines that initiate pyroptosis, an inflammatory form of programmed cell death [8-11].
In humans, dysregulation of NLRP3 inflammasome signaling by gain-of-function mutations in coding regions results in cryopyrin-associated periodic syndromes (CAPS), which are characterized by inflammatory conditions in the lungs, skin, joints, and eyes [12, 13]. Conversely, in the gut, no inflammatory symptoms have been associated; rather the opposite has been observed where the same mutations have led to homeostasis of the gut microbiota and a protective effect against inflammation and CRC [12-14]. One study showed that hyperactive NLRP3 inflammasomes improve gut symbiosis through microbiota [10, 12]. This effect leads to homeostasis of the gut microbiota and produces a protective effect for CRC development [3, 12, 15]. The study proposes a mechanism in which the hyperactivation of NLRP3 inflammasome leads to over-production of IL-1β but not IL-18 [10, 12, 16].
Considering the symbiotic effects of H. influenzae on the microbiome, we postulated that a history of infection with this pathogen leads to a decreased risk of CRC. This study aimed to evaluate the correlation between H. influenzae infection and CRC incidence.
Materials and Methods
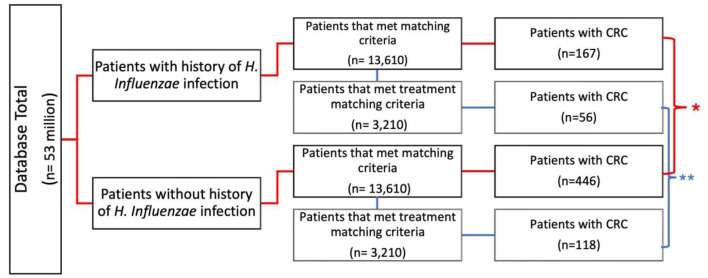
This study was a retrospective cohort analysis. Access to a Humana Health Insurance HIPAA-compliant national database was provided by Holy Cross Health, Fort Lauderdale for the sole intention of academic research. The database utilized called PearlDiver, in conjunction with the Bellwether interface, was used to run queries, stratify data, and perform statistical analyses. PearlDiver contains over 53,000,000 HIPAA-compliant patient records. Patients were selected retrospectively using the International Classification of Disease 9th and 10th Codes (ICD-9, ICD-10), Current Procedural Terminology (CPT), and National Drug Codes to identify all types of CRC diagnoses and previous H. influenzae infection from January 2010 to December 2019. The duration of H. influenzae infection was not recorded and is unknown; however, infection had to precede the diagnosis of cancer to be included. As shown in Figure 1, the data were initially filtered to evaluate patients previously infected with H. influenzae (experimental group) and those who had never been infected with H. influenzae (control group). The control and experimental groups were then matched for age range, sex, and Charlson Comorbidity Index (CCI) to allow for optimal comparison, while mitigating confounding variables on outcome measures. A total of 13,610 patients met the matching criteria for both groups. Following this initial match, the data were further stratified to include antibiotic treatment exposure in both groups, with 3,210 patients in each group. The inclusion criteria for this study were maintaining an active status in the database for at least 8 years and a history of H. influenzae infection that preceded CRC if cancer did develop. The inclusion criteria of the control group included active status in the database for at least 8 years, and no history of H. influenzae infection that preceded if CRC did develop. The primary outcome measure of this study was the development of CRC. The query was analyzed from January 2010 to December 2019 and resulted in 13,610 patients after matching in both experimental and control groups. A Pearson’s Chi-square test with Yates’ continuity correction and odds ratio (OR) were performed; this was to determine the significance of potential differences in incidence of CRC between populations with and without a history of H. influenzae infection. Significance was assessed using relative risk with a 95% confidence interval (CI), and the PearlDiver statistical software was used to calculate all data outcomes. Subsequent analysis of the data included a breakdown of patient demographics by age, sex, and region (Figs. 2-4).
Figure 1.
Flowchart of infected vs. non-infected groups and incidence of CRC. Evaluation of incidence of CRC of patients with and without history of H. influenzae infection (control) (*P < 2.2 × 10-10, **P = 3.5 × 10-13). Additional stratification and matching were completed for treatment. CRC: colorectal cancer; H. influenzae: Hemophilus influenzae.
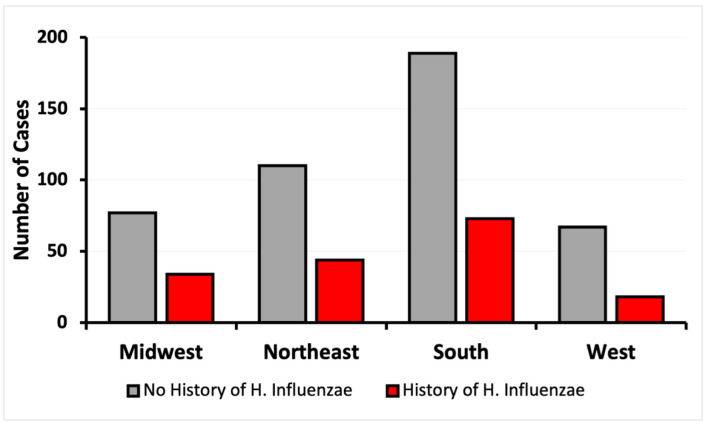
Figure 2.
Incidence of CRC by regions of residence of patients with and without exposure to H. influenzae. CRC: colorectal cancer.
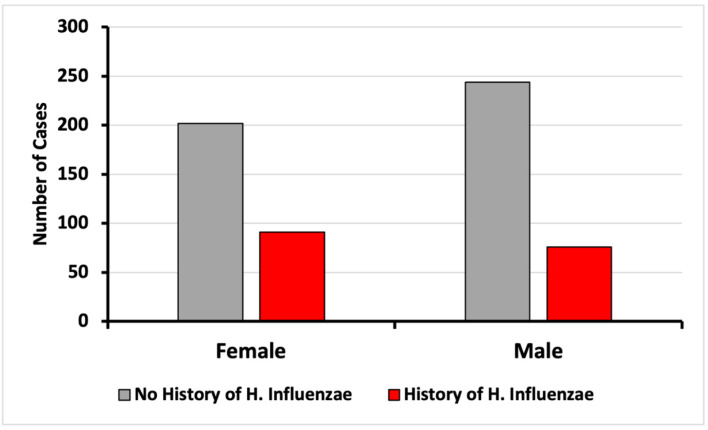
Figure 3.
Incidence of CRC based on sex on patients with and without exposure to H. influenzae. CRC: colorectal cancer.
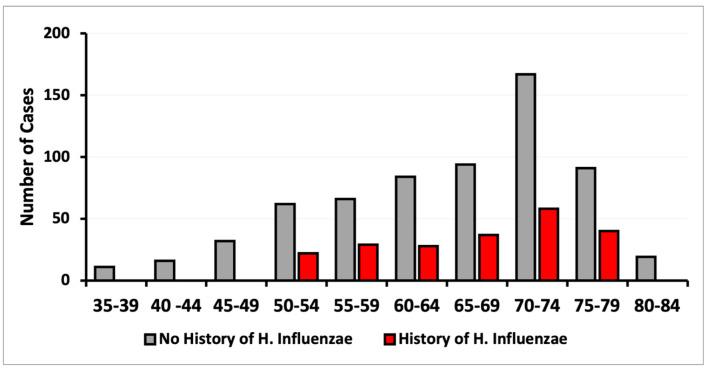
Figure 4.
Incidence of CRC based on age of diagnosis on patients with and without exposure to H. influenzae. CRC: colorectal cancer.
Literature review
PubMed was used for literature review. The literature review aimed to collect data related to any and all relationships between CRC and H. influenzae infection. The review was guided using keywords in varying orders: “colorectal cancer”, “Hemophilus influenzae infection”, “microbiome”, “carcinogenesis”, “immunology”, and “bacterial infection”. The literature includes findings relating to the microbiome and its impact on carcinogenesis and CRC.
Institutional Review Boards (IRB) approval
This study was exempt from IRB approval because all data were obtained from a database that provided de-identified patient information. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Results
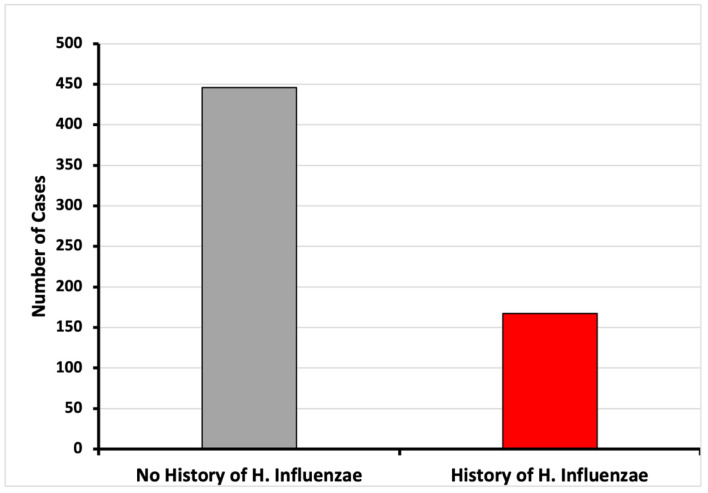
A database of 53 million patients was used to identify those with and without a history of H. influenzae. A total of 13,610 patients met the matching criteria for age range and Charlson Comorbidity Index (CCI) and were assigned to both the control group (without history of H. influenzae infection) and the experimental group (with a history of H. influenzae infection). In the control group, 446 patients (3.23%) without H. influenzae infections were diagnosed with CRC. In contrast, only 167 (1.22%) patients with a history of H. influenzae infections were diagnosed with CRC. This difference was statistically significant, with a P-value of < 2.2 × 10-16 and an OR of 0.41 (95% CI: 0.36 - 0.47) (Figs. 1 and 5).
Figure 5.
Incidence of CRC of patients with and without history of H. influenzae infection (*P < 2.2 × 10-16; OR = 0.41, 95% CI: 0.36 - 0.47). CI: confidence interval; CRC: colorectal cancer; OR: odds ratio.
These 13,610 patients were further evaluated and matched for exposure to antibiotic treatment. The incidence of CRC was observed in 56 patients (1.7%) in the H. influenzae group compared to 118 patients (3.7%) in the control group (P = 3.5 × 10-13; OR = 0.51, 95% CI: 0.43 - 0.62 (Fig. 1)).
The demographics of matched patients with and without a history of H. influenzae infection were analyzed. The data were stratified based on age at CRC diagnosis, sex, and region of residence. Four regions were considered: Midwest, Northeast, South, and West. The control group showed a higher incidence of CRC in all regions, with the highest prevalence of CRC in the southern region (Fig. 2). All regions showed a decrease of at least 2.3-fold in CRC risk in patients exposed to H. influenzae infection (Fig. 2).
Upon analysis of CRC incidence by sex, no differences were found in this study (Fig. 3). The diagnosis of CRC was more prevalent in both the control and infected groups within the age range of 50 to 79 years. The data demonstrated a peak CRC incidence in patients aged between 70 and 74 years (Fig. 4). When stratified by age and sex, the data also showed a lower incidence of CRC in patients with a history of H. influenzae infection.
Discussion
Previously published data on H. influenzae have linked more common cancers to this microorganism [17-19]. However, there are no current data on the relationship of this pathogen to CRC, making this study the first, to date, to investigate the link between previous history of H. influenzae infection and the development of CRC. This study established a statistically significant correlation between H. influenzae infection and a reduced incidence of CRC.
This study illustrates the complex nature and pathogenesis of H. influenzae, a bacterium initially believed to be involved in chronic inflammatory states, such as in the development of chronic obstructive pulmonary disease (COPD) and lung tumorigenesis [17-19]. This study sheds light on the paradoxical nature of H. influenzae, which induces a protective effect in CRC. It is hypothesized that activation of the NLRP3 inflammasome leads to a strongly driven IL-1β inflammatory response, that is, the lung leads to the pathogenesis of lung diseases such as COPD and protracted bacterial bronchitis (PBB) [7, 20-22]. In contrast, similar effects in the gut might lead to microbiota homeostasis and prevention of CRC as demonstrated by the results of this study. One study proposed a mechanism in which the hyperactive NLRP3 inflammasome leads to overproduction of IL-1β, but not IL-18, causing an improvement in gut symbiosis. The homeostasis of the gut microbiota then leads to a protective effect for CRC development [10, 12, 15, 16].
Growing evidence has shown a key role for inter-kingdom crosstalk in maintaining host homeostasis and disease evolution, specifically in the gut-lung axis (GLA) [23-25]. This paper highlights the interaction between H. influenzae, a pathogen commonly associated with the upper respiratory tract, and its relationship with the gut by reducing the incidence of CRC. Making an emphasis on GLA microbiota and disease pathogenesis prevention rather than the previously thought chronic inflammatory state such as findings like COPD patients have been shown to be 2 - 3 times more likely to be diagnosed with IBD [24, 26].
This study has some limitations. First, this was a single-center retrospective review that included patients from a specific location. Additionally, given the retrospective nature of our study, we did not have data regarding the patient population or specific details regarding H. influenzae infection. Additionally, the widespread childhood immunization program for H. influenzae caused a decrease in the incidence of infections, limiting the number of patients available for the study. Other limitations include the lack of H. influenzae testing if the patient did not have any symptoms that could indicate the order of the test to localize the pathogen related to the suspected infectious disease. Therefore, there could be a decrease in the number of patients who overlap in the workup of H. influenzae infection and the diagnosis of CRC. Our study suggests that pathogens such as H. influenzae are involved not only in the acute microbiome but also in more chronic changes in the human microbiota. These changes may affect the development of CRC, as in our study, but may also affect or potentiate the development of other types of cancer, such as lung cancer [7]. Future studies should address other pathogens and their relationship with the gut microbiota. Understanding how microorganisms such as H. influenzae, via inflammasome upregulations, lead to dysbiosis or symbiosis of the microbiota, might elucidate a better understanding of the pathogenesis of CRC and potential therapeutic targets such as the inflammasome pathway [3].
It is important to evaluate previous immunizations used for Hib prevention to analyze whether the same inflammatory response is induced by vaccination. A common formulation of the Hib vaccine is a conjugated vaccine consisting of a capsular polysaccharide (PS) covalently linked to proteins. Among these formulations, Hib-outer membrane protein complex (OMPC) was shown to engage Toll-like receptor 2 (TLR2) and enhance the immunogenicity of the Hib PS [27]. Nonetheless, Hib conjugated with tetanus toxoid and cross-reacting material (CRM) did not show any relationship with TLR2 [27, 28]. On the other hand, other potential targets to be studied in the context of incidence of CRC include vaccine adjuvants that target the NLRP3 inflammasome. Some of these include aluminum salt-based adjuvants, saponin-based adjuvants, emulsion-based adjuvants, and TLR agonists [28, 29]. Vaccines incorporate TLR ligands not only to protect against infectious diseases but also as a therapeutic immunization against noninfectious diseases such as cancer [30]. Specifically, 3-O-desacyl-4-monophosphoryl lipid A (MPL) has been used as an adjuvant in clinical trials of vaccines against multiple cancers including CRC, melanoma, glioma, and pancreatic cancer. Nonetheless, the trials were not able to show significant clinical effects but demonstrated tumor-specific immunity in response to immunization [27]. It is important to consider future studies to test adjuvants usually used in the formulation of vaccines that target the NLRP3 inflammasome or Hib vaccines and to assess if there is a protective effect against CRC.
The next step in the study could be to further analyze the data by reversing the order of acquisition. First, obtaining, and matching data based on patients’ diagnosis of CRC and then evaluating how many of the patients in each group had or did not have a concomitant H. influenzae infection. In this manner, we examine data from the opposite direction.
Ultimately, future studies are crucial to confirm the mechanism of our results and to characterize the precise mechanism and pathogenesis by which H. influenzae may reduce the risk of CRC. Targeting these inflammatory pathways may lead to the development of novel therapeutics capable of reducing the risk of one of the deadliest cancers in the world, and potentially serve as a therapy for other cancers.
Conclusions
The study shows a statistically significant correlation between history of H. influenza infection and reduced CRC incidence. Although the study design cannot determine causality, H. influenzae infection causes the upregulation of the NLRP3 inflammasome. In contrast, the NLRP3 inflammasome has been linked to a protective effect on gut microbiota homeostasis and a reduction in the risk of CRC. It is paramount to investigate this relationship to understand and assess the role of NLRP3 as a potential target for onco-protection against CRC, and to understand the mechanism of H. influenzae in reducing the CRC incidence.
Acknowledgments
The authors acknowledge the support of Holy Cross Health and Nova Southeastern University Dr. Kiran C. Patel College of Allopathic Medicine.
Financial Disclosure
Rashid OM has received peer reviewed grant funding from the Community Foundation of Broward.
Conflict of Interest
None to declare.
Informed Consent
Exempt from IRB approval due to use of de-identified national database.
Author Contributions
Author Marla C. Fortoul is responsible for manuscript writing and literature review. Lexi Frankel and Amalia Ardeljan are responsible for inputting queries, database data extraction, and data analysis. Enoch Kim is responsible for manuscript and figure editing. Authors Kazuaki Takabe and Omar Rashid are responsible for manuscript writing, editing, and data analysis.
Data Availability
The data supporting the findings of this study have been extracted from PearlDiver national database.
Abbreviations
- CCI
Charlson Comorbidity Index
- COPD
chronic obstructive pulmonary disease
- CRC
colorectal cancer
- GLA
gut-lung axis
- IBD
inflammatory bowel disease
- MPL
3-O-desacyl-4-monophosphoryl lipid A
- NLRP3
nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3
- NTHi
non-typeable H. influenzae
- OMPC
outer membrane protein complex
- PBB
protracted bacterial bronchitis
- PS
polysaccharide
- TLR
toll-like receptor
References
- 1.Brody H. Colorectal cancer. Nature. 2015;521(7551):S1. doi: 10.1038/521S1a. [DOI] [PubMed] [Google Scholar]
- 2.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 3.Rastogi YR, Saini AK, Thakur VK, Saini RV. New insights into molecular links between microbiota and gastrointestinal cancers: a literature review. Int J Mol Sci. 2020;21(9):3212. doi: 10.3390/ijms21093212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong D, Dregan A, Ashworth M, White P, McGee C, de Lusignan S. The association between colorectal cancer and prior antibiotic prescriptions: case control study. Br J Cancer. 2020;122(6):912–917. doi: 10.1038/s41416-019-0701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, Li Y, Lam V. et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2011;17(6):1359–1372. doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh V, Nanjappa S, Pabbathi S, Greene JN. Invasive Haemophilus influenzae Infection in Patients With Cancer. Cancer Control. 2017;24(1):66–71. doi: 10.1177/107327481702400111. [DOI] [PubMed] [Google Scholar]
- 7.Rotta Detto Loria J, Rohmann K, Droemann D, Kujath P, Rupp J, Goldmann T, Dalhoff K. Nontypeable haemophilus influenzae infection upregulates the NLRP3 inflammasome and leads to caspase-1-dependent secretion of interleukin-1beta - a possible pathway of exacerbations in COPD. PLoS One. 2013;8(6):e66818. doi: 10.1371/journal.pone.0066818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karki R, Man SM, Kanneganti TD. Inflammasomes and Cancer. Cancer Immunol Res. 2017;5(2):94–99. doi: 10.1158/2326-6066.CIR-16-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30(6):860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265(1):6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao X, Zhang C, Xing Y, Xue G, Zhang Q, Pan F, Wu G. et al. Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nat Commun. 2017;8(1):1896. doi: 10.1038/s41467-017-01917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinarello CA. Mutations in cryopyrin: bypassing roadblocks in the caspase 1 inflammasome for interleukin-1beta secretion and disease activity. Arthritis Rheum. 2007;56(9):2817–2822. doi: 10.1002/art.22841. [DOI] [PubMed] [Google Scholar]
- 14.Gattorno M, Piccini A, Lasiglie D, Tassi S, Brisca G, Carta S, Delfino L. et al. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2008;58(5):1505–1515. doi: 10.1002/art.23437. [DOI] [PubMed] [Google Scholar]
- 15.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32(3):379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngui IQH, Perera AP, Eri R. Does NLRP3 inflammasome and Aryl hydrocarbon receptor play an interlinked role in bowel inflammation and colitis-associated colorectal cancer? Molecules. 2020;25(10):2427. doi: 10.3390/molecules25102427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sriram KB, Cox AJ, Sivakumaran P, Singh M, Watts AM, West NP, Cripps AW. Non-typeable Haemophilus Influenzae detection in the lower airways of patients with lung cancer and chronic obstructive pulmonary disease. Multidiscip Respir Med. 2018;13:11. doi: 10.1186/s40248-018-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moghaddam SJ, Li H, Cho SN, Dishop MK, Wistuba, II, Ji L, Kurie JM. et al. Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model. Am J Respir Cell Mol Biol. 2009;40(4):443–453. doi: 10.1165/rcmb.2008-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barta P, Van Pelt C, Men T, Dickey BF, Lotan R, Moghaddam SJ. Enhancement of lung tumorigenesis in a Gprc5a Knockout mouse by chronic extrinsic airway inflammation. Mol Cancer. 2012;11:4. doi: 10.1186/1476-4598-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen AC, Tran HB, Xi Y, Yerkovich ST, Baines KJ, Pizzutto SJ, Carroll M. et al. Multiple inflammasomes may regulate the interleukin-1-driven inflammation in protracted bacterial bronchitis. ERJ Open Res. 2018;4(1):00130–2017. doi: 10.1183/23120541.00130-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185(8):4912–4920. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L. et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieers G, Guery B, Delhaes L. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15(1):55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 25.Zeng MY, Inohara N, Nunez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10(1):18–26. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012;5(1):7–18. doi: 10.1038/mi.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latz E, Franko J, Golenbock DT, Schreiber JR. Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J Immunol. 2004;172(4):2431–2438. doi: 10.4049/jimmunol.172.4.2431. [DOI] [PubMed] [Google Scholar]
- 28.Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, Persing D. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther. 2004;4(7):1129–1138. doi: 10.1517/14712598.4.7.1129. [DOI] [PubMed] [Google Scholar]
- 29.Reinke S, Thakur A, Gartlan C, Bezbradica JS, Milicic A. Inflammasome-mediated immunogenicity of clinical and experimental vaccine adjuvants. Vaccines (Basel) 2020;8(3):554. doi: 10.3390/vaccines8030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27(1):49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study have been extracted from PearlDiver national database.