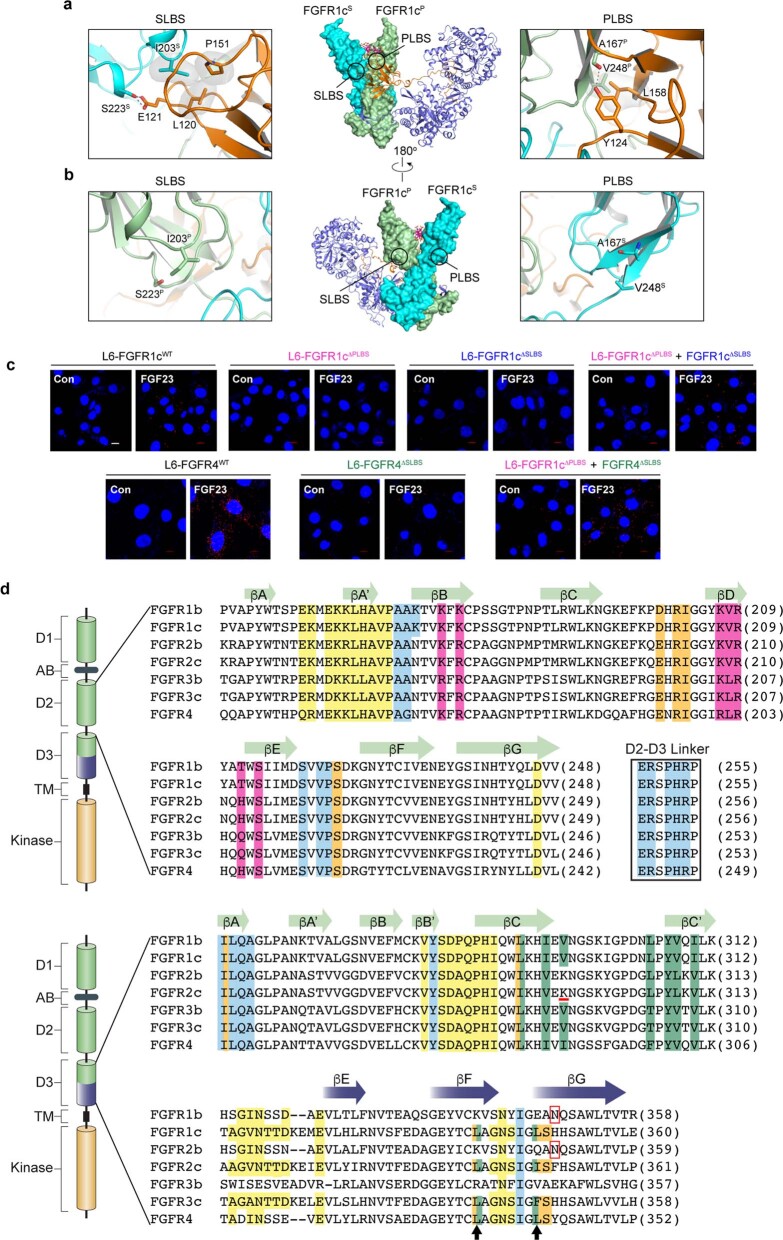
Extended Data Fig. 5. Rationale for cell-based receptor complementation assay and structure-based sequence alignment of seven FGFR isoforms.
a-b, Rationale for receptor complementation assay. Center: Cartoon/surface representation of the FGF23–FGFR1c–αKlotho–HS quaternary complex in two orientations related by 180° rotation around Y axis. Circles denote approximate circumferences of ligand binding sites in primary and secondary receptors selected for mutagenesis. a, Left: expanded view of circled region on the secondary receptor showing that Ile-203 and Ser-223 of FGFR1cS engage in hydrophobic and hydrogen bonding contacts with residues in FGF23’s core. Right: expanded view of circled region on the primary receptor showing that Ala-167 and Val-248 of FGFR1cP engage in highly conserved hydrophobic contacts with Tyr-124 and Leu-158 of FGF23, respectively. Additionally, Ala-167 also makes a hydrogen bond with Tyr-124. b, Left, expanded view of the circled region on the primary receptor showing that the corresponding Ile-203 and Ser-223 in FGFR1cP are solvent exposed. Thus, an engineered FGFR1cI203E/S223E molecule (i.e., FGFR1cΔSLBS) should preserve the ability to act as primary receptor despite losing the capacity to function as secondary receptor. Right, expanded view of the circled region on secondary receptor showing that the corresponding Ala-167 and Val-248 in FGFR1cS do not play any role in the quaternary complex formation and are solvent exposed. Thus, an engineered FGFR1cA167D/V248D mutant (i.e., FGFR1cΔPLBS) is predicted to lose the ability to function as primary receptor but could still act as secondary receptor. c, Demonstration of receptor complementation between FGFR1cΔSLBS and FGFR1cΔPLBS and between FGFR4ΔSLBS and FGFR1cΔPLBS via PLA. Representative fluorescent microscopy fields of stable cell lines subjected to PLA are shown. Scale bar,10 μm. Experiments were performed at least two times biological repeat with similar results. d, Structure-based sequence alignment of ligand binding regions (i.e., D2, D2-D3 linker and D3) of all seven FGFR isoforms. As a guide, a schematic representation of a prototypical FGFR is shown and its various domains are labeled. The C-terminal half of D3 which undergoes alternative splicing in FGFR1-FGFR3 is highlighted in dark blue. Note that the unspliced N-terminal half of D3 is conserved between b and c isoforms. Secondary structure elements are provided on the top of alignment. The HS interacting residues are all localized within D2 domain and are colored in magenta. Residues mediating the FGF23-FGFRP and αKlotho-FGFRP interfaces are colored yellow and green, respectively. Note that residues mediating direct FGFRP-FGFRS contacts (in light blue) are fully conserved amongst all seven isoforms. As for the FGF23-FGFRS interface (in orange), only D2 residues that contact FGF23’s core, are conserved between the seven isoforms. However, two hydrophobic D3 residues that interact with FGF23 N-terminus at the FGF23-FGFRS interface are conserved only in FGFR1c-3c and FGFR4 (indicated by black arrow heads). In FGFR1b-3b, these residues, which overlap with binding site for RBA of αKlotho, are replaced by charged residues. Hydrophobicity of D3 groove FGFR1b-3b is further disrupted by the presence of a unique N-linked glycosylation site within their D3 grooves (denoted by a red box). In FGFR2c, a key conserved hydrophobic residue is replaced by a charged Lys-296 (underlined in red) which likely accounts for the inability of FGFR2c to bind αKlotho.