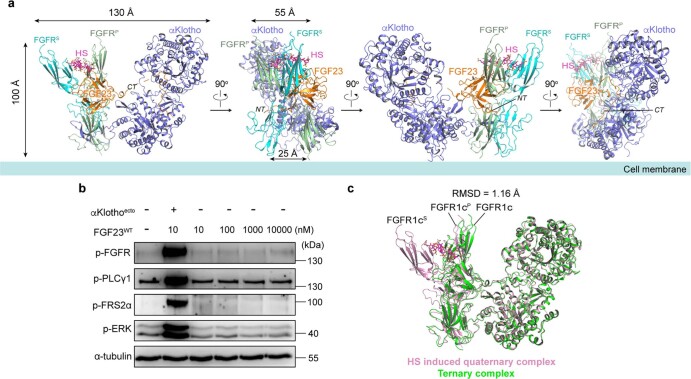
Extended Data Fig. 2. Overall topology of the FGF23 signaling complex and its strict αKlotho dependency.
A, Cartoon representation of the cryo-EM structure of the 1:2:1:1 FGF23-FGFR-αKlotho-HS quaternary complex in four different orientations related by 90° rotation along the vertical axis. The asymmetric quaternary complex has an average dimension of 130 Å × 100 Å × 55 Å. The proximity of membrane insertion points of FGFR chains (~25 Å apart) would be conducive to the formation of an asymmetric A-loop trans-phosphorylating dimer of intracellular kinase domain27. FGF23, αKlotho and HS are shown in orange, blue and magenta, respectively. Primary receptor (FGFRP) and secondary receptor (FGFRS) are shown in pale green and cyan. Dashed lines denote residues 172–182 of FGF23, the linker between FGF23’s trefoil core and its distal αKlotho binding site, which could not be built due to lack of interpretable electron density. This region does not interact with either αKlotho or FGFR and is likely disordered/flexible. Notably, this region harbors the regulatory subtilisin-like proprotein convertase (SPC) site,176RHT178R179/S180AE182, which includes a furin type protease cleavage site (R179), an O-glycosylation site (T178) and a serine phosphorylation site (S180). Three enzymes namely GalNAc-T3(N-acetylgalactosaminyltransferase3), Fam20C (the family with sequence similarity 20, member C), and a yet to be discovered furin type protease converge on this site to regulate FGF23 processing. The high flexibility of this region is likely necessary for the action of these enzymes. B, FGF23 signaling is strictly αKlotho dependent. L6-FGFR1cWT cells were treated with increasing concentrations of recombinant FGF23WT alone, in combination with soluble αKlotho or left untreated. Whole cell lysates immunoblotted as in Fig. 2c. Note that even supra pharmacological concentrations (as high as 10 micromolar) of FGF23WT fails to activate FGFR1c signaling. However, when co-treated with soluble αKlotho, as little as 10 nM FGF23 induces a robust FGFR1c activation. Experiments were performed in biological triplicates with similar results. C, Superimposition of X-ray structure of the FGF23–FGFR1c–αKlotho ternary complex (PDB ID: 5W21, colored in green) onto the corresponding FGF23–FGFR1cP–αKlotho portion within the cryo-EM structure of 1:2:1:1 FGF23-FGFR1c-αKlotho-HS quaternary complex (colored in pink) shows high degree of similarity (RM)D of 1.16 Å].