Abstract
This study aimed to optimize methods for identifying heat-tolerant and heat-susceptible cotton plants by examining the relationship between leaf physiology and cotton yield. Cotton accessions were exposed to elevated temperatures through staggered sowing and controlled growth conditions in a glasshouse. Based on their yield performance, leaf physiology, cell biochemistry, and pollen germination, the accessions were categorized as heat-tolerant, moderately tolerant, or susceptible. High temperatures had a significant impact on various leaf physiological and biochemical factors, such as cell injury, photosynthetic rate, stomatal conductance, transpiration rate, leaf temperature, chlorophyll fluorescence, and enzyme activities. The germination of flower pollen and seed cotton yield was also affected. The study demonstrated that there was a genetic variability for heat tolerance among the tested cotton accessions, as indicated by the interaction between accession and environment. Leaf gas exchange, cell biochemistry, pollen germination, and cotton yield were strongly associated with heat-sensitive accessions, but this association was negligible in tolerant accessions. Principal component analysis was used to classify the accessions based on their performance under heat stress conditions. The findings suggest that leaf physiological traits, cell biochemistry, pollen germination, and cotton yield can be effective indicators for selecting heat-tolerant cotton lines. Future research could explore additional genetic traits for improved selection and development of heat-tolerant accessions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-023-01322-8.
Keywords: Cotton, Temperature extreme, Cell injury, Genetic variability, PS-II, Yield
Introduction
The global atmospheric temperature is rising steadily because of changing climates (Ababaei and Chenu 2020). For instance, a 1.5 °C rise in global atmospheric temperature has been recorded during the last decade (Bel and Teixidó 2020), with a further projection of a 2.6 °C rise by 2050 and a 5.8 °C by 2100 (Stocker 2014). Cotton crops, which are commonly grown in warm subtropical regions, encounter elevated temperatures during their reproductive phase. Therefore, screening cotton accessions against heat stress could be utilized to identify novel germplasm for high-temperature areas (Salman et al. 2019). Different screening tools were used during vegetative and reproductive growth stages for screening heat-tolerant cotton accessions (Singh et al. 2018). Relative cell injury, chlorophyll fluorescence, and gas exchange components (net photosynthetic rate, stomatal conductance, and transpiration rate) are the reliable and easy physiological indexes for screening heat-tolerant and heat susceptible accessions under stressful environments (Mantri et al. 2014).
High temperature strongly influences various physiological processes, e.g. membrane stability, photosynthesis, and stomatal conductance of cotton crops, leading to lint yield penalties (Cottee et al. 2010; Najeeb et al. 2017; Sarwar et al. 2018, 2019). Damage to physiological functioning is associated with heat-induced injury to the lipid membrane of cellular organelles (Najeeb et al. 2011; Abro et al. 2015; Masoomi-Aladizgeh et al. 2021). High temperatures break hydrogen bonds and electrostatic interactions between the polar groups of proteins in the aqueous phase of the membrane, altering the composition of cellular membranes (Rehman et al. 2015). For example, previous studies suggested membrane damage to cotton as the atmospheric temperature increased from 33 to 35 °C (Sarwar et al. 2017; Azhar et al. 2009). Damage to the lipid membrane affects plant functioning, especially the carbon assimilation process, i.e., photosynthesis inhibition is regarded as the earliest sign of heat-induced injury in cotton plants (Karademir et al. 2018). The favorable temperature for photosynthesis in cotton is 30–32 °C, beyond which it deleteriously affects the photosynthetic parameters (Reddy et al. 1999; Wang et al. 2011; Loka et al. 2020). For instance, a decrease in photosynthesis in some cotton accessions has been recorded at 36 °C (Bibi et al. 2008) with complete inhibition at around 45 °C (Najeeb et al. 2017; Sarwar et al. 2019). This inhibited photosynthesis could be the result of stomatal closure and/or reduced PSII efficiency (Masoomi-Aladizgeh et al. 2021). Maximum photosynthesis and quantum yield of PS-II occurs at 33 °C; while a temperature of 36 °C or above can significantly reduce the quantum yield of PS-II (Brown and Oosterhuis 2004; Chastain et al. 2016).
Increased temperature negatively impacts various reproductive processes in plants, such as pollen formation, germination, and fertilization, leading to the shedding of vegetative and reproductive tissues (Kakani et al. 2005). For instance, temperatures exceeding 35 °C can cause irreversible damage to pollen cell membranes, reducing their viability and germination potential (Min et al. 2014). Heat-tolerant cotton genotypes demonstrate superior pollen germination and fertilization capabilities under high-temperature stress compared to heat-susceptible genotypes (Singh et al. 2018). Consequently, the inadequate development of reproductive structures like pollen and pistil contributes to yield losses in heat-stressed cotton crops (Zhao et al. 2005). High temperatures disrupt the physiological functioning of plants, resulting in diminished growth and yield. Field crops, including cotton, experience an imbalance between reactive oxygen species (ROS) and the plant's defense system when exposed to high temperatures (Sarwar et al. 2019). Heat-induced membrane leakage during flowering in cotton under hot environments leads to oxidative damage, impairing physiological functions such as photosynthesis (Pn), stomatal conductance (Gs), and chlorophyll fluorescence (Fv/Fm) (Snider et al. 2009). Cotton varieties that possess effective antioxidant systems, maintain photosynthesis rates (Pn), and sustain stomatal conductance (Gs) exhibit greater resilience to heat stress (Singh et al. 2018).
Pakistan's April/May sown cotton crop experiences high temperatures during its reproductive stages, particularly at the peak flowering stage, which directly impacts cotton lint yield. Heat waves hasten the shedding of developmental structures and significantly reduce crop productivity (Burke and Wanjura 2010; Schlenker and Roberts 2009). Previous studies have utilized principal component analysis (PCA) to identify genetic diversity among cotton accessions based on agronomic traits and group them accordingly (Li et al. 2008; Qiaoling and Zhe 2011; Jarwar et al. 2019). Ullah et al. (2020) assessed the genetic diversity of cotton accessions using PCA, considering attributes such as sympodial branches, bolls per plant, boll weight (BW), yield per plant, plant height, and seed index, where PCA-I and PCA-II encompassed the maximum variation across these attributes. Demİrel et al. (2016) found a positive association between cotton physiological traits and seed cotton yield, suggesting PCA-based selection of accessions using physiological and yield traits as a reliable technique. Similarly, in maize and cotton, PCA based on physiological traits proved effective for identifying genetic variation under heat and drought stress (Naveed et al. 2014; Ullah et al. 2019).
Considering the significance of high-temperature stress on cotton crops, this study was conducted under glasshouse and field conditions to evaluate the effects of temperature on cotton accessions, aiming to identify heat-tolerant and susceptible genotypes. The study had two objectives: first, to assess and compare the performance of different cotton accessions under terminal heat stress during flowering using various parameters, including leaf gas exchange components, leaf temperature, membrane leakage, pollen germination, cell biochemistry, and seed cotton yield, to develop a more effective screening technique for identifying heat-tolerant genotypes. Second, the study aimed to investigate the relationships between the different parameters studied using regression, correlation, and principal component analysis for both heat-tolerant and heat-susceptible genotypes (Tables 1 and 2).
Table 1.
Physio-chemical analysis of glass house soil during 2011
| Characteristics | Unit | Value |
|---|---|---|
| Soil | Peat to silt ratio 3:1 | |
| EC | dS/m | 0.56 |
| Ph | – | 8.3 |
| Organic matter | % | 1.34 |
| Available phosphorus | Ppm | 28.5 |
| Available potassium | Ppm | 245 |
| Available zinc | Ppm | 1.5 |
| Available boron | Ppm | 0.8 |
Table 2.
Physio-chemical analysis of field soil during 2011
| Characteristics | Unit | Value | |
|---|---|---|---|
| Depth of sample | cm | 0–6 | 6–12 |
| Sand | % | 51 | 50 |
| Silt | % | 22 | 21 |
| Clay | % | 27 | 29 |
| Textural class | Sandy clay loam | ||
| Saturation | % | 31 | 30 |
| EC | dS/m | 1.5 | 1.3 |
| Ph | – | 8.1 | 8.1 |
| Organic matter | % | 0.43 | 0.32 |
| Total nitrogen | % | 0.031 | 0.026 |
| Available phosphorus | Ppm | 7.2 | 6.1 |
| Available potassium | Ppm | 235 | 207 |
| Available zinc | Ppm | 1.4 | 1.2 |
| Available boron | Ppm | 0.8 | 0.7 |
Total nitrogen, i.e. organic N + inorganic N
Materials and methods
Glasshouse experiment
Cotton germplasm for heat tolerance
Thirty cotton (Gossypium hirsutum L.) accessions were collected from national research organizations as parental resources for heat-tolerance screening (Table S1). These accessions represented commercially cultivated varieties in Pakistan. The plants were grown under two temperature regimes in the glasshouse and two thermal regimes in the field. In the glasshouse, 34/21 ± 2 °C was the optimal temperature, while 45/30 ± 2 °C represented high-temperature stress during flowering. The early-sown crop in April experienced high temperatures during flowering in June, while the late-sown crop in June faced low/optimal temperatures during flowering (Table 3).
Table 3.
Variable temperatures at flowering of cotton during one week of study under field conditions
| 5th April sown crop | 17th June sown crop | ||||||
|---|---|---|---|---|---|---|---|
| Days of the year | Tmax (°C). ambient | Tmin (°C). ambient | R.H. (%) | Days of the year | Tmax (°C). Ambient | Tmax (°C). of polythene sheet | R.H. (%) |
| 27-May | 44.1 | 25.0 | 18 | 15-August | 27.0 | 23.7 | 88 |
| 28-May | 45.4 | 27.2 | 17 | 16-August | 30.2 | 24.2 | 86 |
| 29-May | 46.3 | 27.5 | 16 | 17-August | 31.3 | 25.0 | 78 |
| 30-May | 44.7 | 25.3 | 18 | 18-August | 32.4 | 26.4 | 74 |
| 31-May | 44.6 | 24.0 | 18 | 19-August | 29.6 | 24.0 | 82 |
| 1-June | 45.5 | 25.1 | 16 | 20-August | 30.1 | 24.9 | 80 |
| 2-June | 44.8 | 24.7 | 19 | 21-August | 31.0 | 25.5 | 77 |
Growth conditions
The glasshouse experiment was conducted at the Department of Plant Breeding and Genetics, University of Agriculture Faisalabad, Pakistan. Cotton accessions were grown under a 14/10 h day/night photoperiod. The seeds were planted in earthen pots (35 × 30 cm in length and width), each filled with 8 kg of soil, with a ratio of 3:1 to peat and silt. In pots, at 30, 60, and 90 days after sowing (DAS), urea solution was applied to a concentration of 12 g/L. In each pot, four seeds were planted, and the thinning was performed at the four-leaf level, leaving only one plant in each pot.
Glasshouse conditions and experimental design
The entries were grouped with a split layout in a completely randomized design. Each entry was expressed by six pots in each of the two chambers (24 plants, 4 per replication). The plants were grown under optimum temperature, i.e., 34/21 ± 2 °C up to flowering (i.e. for 50 days), and then one set of pots was shifted to a hot chamber, where the air temperature was gradually increased to 2 °C per day up to 45/30 ± 2 °C. The plants were kept at 45/30 ± 2 °C during flowering. Fluorescent bulbs were used to supplement light, although sunlight was the primary source of illumination in the chambers (1400–1600 µmol m−2 s−1 PAR). Relative humidity in all chambers varied between 64 and 80%. During vegetative growth, the pots were irrigated in the morning on alternate days but watered daily during the period of heat stress to avoid water stress.
Data collection
At the end of heat stress (7 d heat), data from the topmost leaves were collected, i.e., relative cell injury (RCI%), net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (E), chlorophyll fluorescence (Fv/Fm), leaf temperature (LT), pollens viability, superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and malondialdehyde (MDA) contents were measured and the pots were returned to optimum temperature room. The glasshouse experiment was terminated after 120 days of sowing, and the data for SCY per plant were collected.
Conditions for the field experiment
Cultural practices
The field experiment was conducted at the Department of Agronomy's Students Research Farm, University of Agriculture Faisalabad, Pakistan. Sandy clay loam was the soil of the experimental area. By manual dibbling, the crop was planted on 75 cm ridges, and a plant distance of 30 cm was maintained. The crop was fully irrigated to avoid multiple stress effects of heat and drought.
Treatment application and experimental design
To grow crops under distinct thermal regimes, planting at different times has been practised (Pettigrew 2002). Two sowing times were selected in this experiment, i.e. April 5 and June 17 during 2011, to provide two temperature regimes (optimum and supra-optimal) at the flowering stage (keeping in view the previous five years’ climate data) (Fig. 1). June thermal regime (late sown crop) was called control because, at the flowering stage, this sowing date faces favorable temperature, while the flowering stage of April (early sowing) faces high temperature. Treatments were arranged in a randomized complete block design with a split-plot layout, having the seeding time (April and June) in the main plots and accessions in sub-plots (Table S2). The experiment was repeated three times using a 6.0 × 4.5 m net plot size.
Fig. 1.
Daily maximum and minimum temperatures of April and June thermal regimes during the cotton growing seasons were statistically different at P < 0.01 when Tmax and Tmin of both sowing dates were tested under t-test
Data collection
Meteorological data were collected throughout the study period from the Agronomy Department's meteorological observatory, the University of Agriculture Faisalabad, Pakistan (Fig. 1). Leaf physiological traits such as relative cell injury (RCI), gas exchange, net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (E), leaf temperature (LT), chlorophyll fluorescence (Fv/Fm), pollens viability, superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and malondialdehyde (MDA) contents were measured from the topmost subtended leaves, one week after heat stress both under glasshouse and field conditions. Leaves were collected between 9:00 and 13:00 h using LCi Analyzer with Wide Head, Part Number LCi-002/B with Serial Number 32455. Measurements of gas exchange were taken at photosynthetically active radiation (PAR) of 1800 µmol photon m2 s1 and a CO2 concentration of 400 µmol mol−1. The temperature inside the leaf chamber was modified based on the corresponding treatment temperature, such as 35 or 45 °C (Fig. S1a and b). To measure RCI, leaf disk samples (10 mm in diameter) were collected. The samples were washed 3–4 times with double distilled water and finally poured into de-ionized water in test tubes. The tubes were incubated at 50 °C (heat treatment) and 25 °C (control) for one hour and then mounted at 25 °C to measure initial electrical conductivity (EC) using an EC meter (Model, Jenway 4510, Japan). After autoclaving the samples (Model, HAU-85., Hirayama instruments, Japan), the final EC was determined at 0.1 MPa pressure for 10 min.
RCI% was recorded according to the formula (Sullivan 1972).
where T1 and T2 are the initial and final EC values of the heat-treated vials, respectively, whereas C1 and C2 are the initial and final EC values of control vials. Cell membrane thermo-stability (CMT) was computed by deducting values out of 100. Gas exchange components, including net photosynthetic rate (Pn, μmol m−2 s−1), stomatal conductivity (Gs, mmol m−2 s−1), transpiration rate (E mmol H2O m−2 s−1), and leaf temperature (°C) were measured using an infrared gas analyzer (LCi Analyzer with Wide Head, Part Number LCi-002/B with Serial Number 32455). Plants were held for one week at a high-temperature chamber after reaching the necessary thermal regime, and data were taken for gas exchange components after that, all pots were moved to the optimum temperature chamber. Thylakoid membrane stability was assessed by measuring chlorophyll fluorescence using a Multi-mode Chlorophyll fluorometer (Opti Science, the UK with Serial Number 0729501) after 20 min of dark adaptation of leaves. Maximum fluorescence (Fv/Fm) was calculated as an indicator of plant stress (Prasad et al. 2008) (Fig. S1 c and d). Seed cotton was harvested 150 days after sowing from all entries. On the same day, seed cotton was carefully selected by hand, avoiding waste and dried carpels. Plot-based calculation of seed cotton yield (SCY), converted to per plant and expressed in grams. For in vitro pollen germination, flowers were collected for pollen viability assays from the first fruiting position one week after the imposition of heat stress both from heat stress and normal conditions of glasshouse and field studies. Between 8:00 and 9:00 a.m., pollen was collected and directly sprinkled on solidified nutrient media (Taylor 1972), containing 1.5 g agar with 30 g sucrose, and 5.3 mg KNO3, 51.7 mg MnSO4, 10.3 mg H3BO3, 10.3 mg MgSO4·7H2O in 100 mL of deionized water (Kakani et al. 2005). Three plants of each genotype were selected, and three flowers from each plant were collected. The pollen germination percentage was calculated and averaged (Fig. S2 a–c). For biochemical attributes, at the end of the heat stress treatment, six leaves were gathered from each plot and three leaves from each pot for biochemical analysis (Fig. S2 d–f). A combined sample was then created by combining the leaves from each plot and pot. To extract enzymes and reactive oxygen species, a 0.5 g leaf sample was obtained from the combined sample. The activity of superoxide dismutase (SOD), measured in U mg−1 protein, was determined by inhibiting nitro blue tetrazolium. An ELISA plate was utilized to contain a reaction mixture of 100 µL enzyme extract, and the absorbance was measured at 560 nm using an ELISA plate reader (Giannopolitis and Ries 1977). The quantification of leaf peroxidase (POD) activity (expressed as U mg−1 protein) was carried out using the guaiacol oxidation procedure. To measure the enzyme quantity at 470 nm, 150 µL enzyme extract was added to the reaction mixtures, which were placed in an ELISA plate (Liu et al. 2009). The catalase (CAT) activity (expressed as U mg−1 protein) was quantified using the protocol described by (Liu et al. 2009), which involved using hydrogen peroxide as a reactant. A reaction mixture consisting of 150 µL enzyme extract was placed in an ELISA plate reader, and the absorbance was recorded at 240 nm. The leaf malondialdehyde (MDA) contents were determined using the method described by (Cakmak and Horst 1991). In this procedure, a 0.5 g leaves sample was homogenized with 3 mL of 0.1% (w/v) trichloroacetic acid (TCA), and the resulting supernatant was collected. To 0.5 mL of supernatant, 3 mL of 20% TCA solution and 0.5% thiobarbituric acid were added. The mixture, along with a blank, was then placed in an ELISA plate, and the absorbance was recorded at 532 and 600 nm.
Data analysis
A two-way analysis of variance was used to assess the individual and interactive effects of heat levels × genotypes under normal and heat stress conditions of glasshouse and field studies. Data at P ≤ 0.01, and P ≤ 0.05 were analyzed statistically using Fisher’s analysis of variance technique (Steel and Torrie 1960). Tukey’s honestly significant difference (Tukey’s HSD) test was employed to compare the means of bar graphs at 1 and 5% using STATISTIX 10.1 software (Gomez and Gomez 1984). Bar graphs and regression graphs were drawn using MS Excel-2016, and the correlation matrix heat maps and PCA analysis were performed in XL-STAT-2014 software. The regression analysis was analyzed statistically under a t-test in MS Excel-2016.
Results
Variations in environmental conditions in the growing season of the trial sites
Temperature regimes (early April and late June) of the field study were significantly different (P ˂ 0.01) when compared under the t-test. Daily maximum (T-max) and minimum temperatures (T-min) from day 1 to 150 of April and June thermal regimes are presented in Fig. 1.
The day maximum temperature at flowering of April sown crops was significantly higher than June sown crops. For example, the April-sown crops experienced a maximum daytime temperature range of 44–46 °C for seven days during their flowering stage, while the June-sown crops faced a maximum daytime temperature range of 27–32 °C during their flowering stage for the same period (Table 3).
Net photosynthetic rate and chlorophyll fluorescence
The heat levels (H) and genotypes (G) showed significant variation under glass house (G × H P ˂ 0.01) and field studies (G × H P ˂ 0.05) for net photosynthetic rate (Pn) and chlorophyll fluorescence (Fv/Fm) (Fig. 2, Table S3–S4). The glass house conditions provided the optimal (34/21 ± 2 °C) and high-temperature stress (45/30 ± 2 °C) at flowering) while the April sowing date of the field study produced the heat stress (44–46 °C), and the June sowing date indicated the optimal temperature (27–32 °C) during one week of flowering. The genotypic performance of thirty accessions for these traits was compared by taking the mean of their means under both thermal regimes of both field studies and the accessions were grouped as heat-tolerant (HT, having mean values above average), moderately heat-tolerant (MHT, having mean values around average) and heat susceptible (HS, having mean values below average) (Table 4).
Fig. 2.
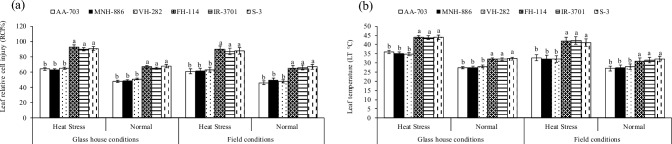
Impact of heat levels and genotypes of glass house (heat levels × genotypes P < 0.01) and field studies (heat levels × genotypes P < 0.05) on a net photosynthetic rate, b chlorophyll fluorescence of three top heat stress tolerant (AA-703, MNH-886, VH-282) and three most heat sensitive (H-114, IR-3701, S-3) cotton genotypes at flowering. Values are the means of three replications (n = 3) ± SE and variants possessing the same letters are not statistically significant at P < 0.01 and P < 0.05). AA-703, MNH-886, VH-282 = heat-tolerant, and FH-114, IR-3701, S-3 = heat susceptible
Table 4.
Mean performance of 30 cotton accessions for net photosynthetic rate (Pn) and chlorophyll fluorescence (Fv/Fm) at flowering stage of cotton in greenhouse and field under two temperature regimes
| Cotton accessions | Pn glasshouse | Pn field | Fv/Fm glasshouse | Fv/Fm field | |||||
|---|---|---|---|---|---|---|---|---|---|
| Stress | Normal | Stress | Normal | Stress | Normal | Stress | Normal | ||
| 1 | C-226 | 26.3 ± 0.6 | 30.3 ± 0.7 | 23.2 ± 1.1 | 32.1 ± 1.6 | 0.71 ± 0.017 | 0.85 ± 0.021 | 0.69 ± 0.034 | 0.77 ± 0.038 |
| 2 | AA-703 | 27.7 ± 0.7 | 32.8 ± 0.8 | 20.5 ± 1.0 | 30.6 ± 1.53 | 0.64 ± 0.016 | 0.73 ± 0.018 | 0.68 ± 0.034 | 0.75 ± 0.037 |
| 3 | MNH-886 | 28.6 ± 0.7 | 32.6 ± 0.8 | 22.5 ± 1.1 | 30.6 ± 1.53 | 0.69 ± 0.017 | 0.79 ± 0.019 | 0.70 ± 0.035 | 0.81 ± 0.04 |
| 4 | CRS-2007 | 24.1 ± 0.6 | 28.1 ± 0.6 | 20.9 ± 1.0 | 27.2 ± 1.36 | 0.65 ± 0.016 | 0.81 ± 0.020 | 0.69 ± 0.034 | 0.82 ± 0.041 |
| 5 | NS-131 | 23.1 ± 0.5 | 27.4 ± 0.6 | 21.6 ± 0.8 | 23.5 ± 1.17 | 0.66 ± 0.014 | 0.67 ± 0.016 | 0.63 ± 0.029 | 0.76 ± 0.035 |
| 6 | KZ-189 | 23.3 ± 0.5 | 27.7 ± 0.7 | 20.4 ± 1.0 | 25.6 ± 1.28 | 0.68 ± 0.017 | 0.76 ± 0.019 | 0.71 ± 0.035 | 0.85 ± 0.042 |
| 7 | FH-172 | 26 ± 0.6 | 29.3 ± 0.7 | 20.9 ± 1.0 | 26.9 ± 1.34 | 0.61 ± 0.015 | 0.75 ± 0.018 | 0.63 ± 0.031 | 0.77 ± 0.038 |
| 8 | VH-282 | 24.6 ± 0.6 | 27.6 ± 0.6 | 18.9 ± 0.9 | 26.8 ± 1.34 | 0.68 ± 0.017 | 0.80 ± 0.020 | 0.66 ± 0.033 | 0.79 ± 0.039 |
| 9 | NS-121 | 24.3 ± 0.6 | 28.7 ± 0.7 | 20.9 ± 1.0 | 27.2 ± 1.36 | 0.64 ± 0.016 | 0.75 ± 0.018 | 0.64 ± 0.031 | 0.78 ± 0.039 |
| 10 | VH-259 | 25.5 ± 0.6 | 28.5 ± 0.7 | 19.5 ± 0.9 | 24.1 ± 1.20 | 0.61 ± 0.015 | 0.76 ± 0.019 | 0.63 ± 0.031 | 0.78 ± 0.039 |
| 11 | AA-802 | 16.2 ± 0.4 | 20.8 ± 0.5 | 12.2 ± 0.6 | 18.5 ± 0.92 | 0.55 ± 0.013 | 0.65 ± 0.016 | 0.56 ± 0.028 | 0.69 ± 0.034 |
| 12 | MNH-888 | 15.7 ± 0.4 | 19.9 ± 0.4 | 13.8 ± 0.6 | 17.8 ± 0.89 | 0.56 ± 0.014 | 0.66 ± 0.016 | 0.59 ± 0.029 | 0.69 ± 0.034 |
| 13 | SB-149 | 15.7 ± 0.4 | 19.1 ± 0.4 | 12.6 ± 0.6 | 17.7 ± 0.88 | 0.57 ± 0.014 | 0.66 ± 0.016 | 0.59 ± 0.029 | 0.69 ± 0.034 |
| 14 | KZ-181 | 14.3 ± 0.3 | 18.3 ± 0.4 | 11.6 ± 0.5 | 15.5 ± 0.77 | 0.55 ± 0.013 | 0.65 ± 0.016 | 0.57 ± 0.028 | 0.67 ± 0.033 |
| 15 | IR-3 | 15.2 ± 0.3 | 18.2 ± 0.4 | 11.5 ± 0.5 | 17.1 ± 0.85 | 0.57 ± 0.014 | 0.66 ± 0.016 | 0.59 ± 0.029 | 0.67 ± 0.033 |
| 16 | FH-142 | 8.7 ± 0.2 | 13.6 ± 0.3 | 8.1 ± 0.4 | 12.1 ± 0.6 | 0.5 ± 0.012 | 0.67 ± 0.016 | 0.6 ± 0.03 | 0.70 ± 0.035 |
| 17 | FH-4243 | 10.5 ± 0.6 | 16 ± 0.4 | 7.4 ± 0.3 | 13.4 ± 0.67 | 0.5 ± 0.012 | 0.63 ± 0.015 | 0.51 ± 0.025 | 0.68 ± 0.034 |
| 18 | IR-3701 | 7.2 ± 0.1 | 11.2 ± 0.2 | 5.1 ± 0.2 | 9.1 ± 0.45 | 0.52 ± 0.013 | 0.63 ± 0.015 | 0.56 ± 0.028 | 0.69 ± 0.034 |
| 19 | FH-113 | 10.3 ± 0.2 | 15.3 ± 0.3 | 7.6 ± 0.3 | 13.5 ± 0.67 | 0.57 ± 0.014 | 0.63 ± 0.015 | 0.59 ± 0.029 | 0.68 ± 0.034 |
| 20 | KZ-191 | 7.1 ± 0.1 | 11.1 ± 0.2 | 5.1 ± 0.2 | 9.2 ± 0.46 | 0.57 ± 0.014 | 0.68 ± 0.017 | 0.56 ± 0.028 | 0.69 ± 0.034 |
| 21 | FH-169 | 11.7 ± 0.3 | 14.7 ± 0.3 | 7.9 ± 0.3 | 12.9 ± 0.64 | 0.57 ± 0.014 | 0.65 ± 0.016 | 0.55 ± 0.027 | 0.69 ± 0.034 |
| 22 | FH-154 | 7.5 ± 0 | 11.6 ± 0.2 | 7.3 ± 0.3 | 11.1 ± 0.55 | 0.53 ± 0.013 | 0.62 ± 0.015 | 0.55 ± 0.027 | 0.60 ± 0.03 |
| 23 | FH-170 | 11.5 ± 0.2 | 14.7 ± 0.3 | 10.6 ± 0.3 | 15.1 ± 0.75 | 0.49 ± 0.012 | 0.66 ± 0.016 | 0.55 ± 0.027 | 0.63 ± 0.031 |
| 24 | VH-148 | 7.4 ± 0.2 | 11.4 ± 0.2 | 7.3 ± 0.3 | 11.9 ± 0.59 | 0.53 ± 0.013 | 0.63 ± 0.015 | 0.58 ± 0.029 | 0.69 ± 0.034 |
| 25 | CRS-456 | 12.7 ± 0.3 | 14.7 ± 0.3 | 9.1 ± 0.4 | 12.9 ± 0.64 | 0.51 ± 0.012 | 0.64 ± 0.016 | 0.59 ± 0.029 | 0.68 ± 0.034 |
| 26 | FH-118 | 7.2 ± 0.1 | 10.8 ± 0.2 | 7.4 ± 0.3 | 11.9 ± 0.59 | 0.53 ± 0.013 | 0.61 ± 0.015 | 0.54 ± 0.027 | 0.64 ± 0.032 |
| 27 | FH-114 | 12.4 ± 0.3 | 16.6 ± 0.4 | 9.8 ± 0.4 | 14.7 ± 0.73 | 0.54 ± 0.013 | 0.6 ± 0.0150 | 0.56 ± 0.028 | 0.63 ± 0.031 |
| 28 | NIAB-820 | 7.5 ± 0.2 | 11.5 ± 0.2 | 7.9 ± 0.3 | 11.4 ± 0.57 | 0.49 ± 0.012 | 0.63 ± 0.015 | 0.51 ± 0.025 | 0.65 ± 0.032 |
| 29 | IR-901 | 7.3 ± 0.2 | 11.4 ± 0.2 | 5.4 ± 0.2 | 9.2 ± 0.46 | 0.51 ± 0.012 | 0.64 ± 0.016 | 0.53 ± 0.026 | 0.67 ± 0.033 |
| 30 | S-3 | 9.3 ± 0.2 | 11.4 ± 0.2 | 6.4 ± 0.3 | 9.1 ± 0.45 | 0.49 ± 0.012 | 0.65 ± 0.016 | 0.51 ± 0.025 | 0.69 ± 0.034 |
| Mean | 15.6 | 19.4 | 12.4 | 17.95 | 0.57 | 0.68 | 0.59 | 0.71 | |
| S.E | 0.39 | 0.48 | 0.63 | 0.89 | 0.014 | 0.017 | 0.029 | 0.035 | |
Average of 30 accessions and SE (R = 3) under normal and stressful conditions of glass house and field
In Table 4, the first 10 cotton accessions were classified as heat-tolerant (HT) because they exhibited above-average Pn and Fv/Fm. The next 5 accessions (11–15) were classified as moderately heat-tolerant (MHT), while the remaining 15 accessions (16–30) were classified as heat susceptible (HS). Principal component analysis (PCA) showed that HT genotypes had higher Pn and Fv/Fm and grouped in the positive quadrate, while HS genotypes with weak Pn and Fv/Fm were grouped in the negative quadrate (Fig. 10). Under high-temperature stress of glasshouse and field studies, the Pn and Fv/Fm values of three HS accessions (FH-114, IR-3701, and S-3) reduced by 54% and 37% (average across) compared to three HT representative genotypes (AA-703, MNH-886, and VH-282) (Fig. 2). The HT accessions also performed 19% better than the HS accessions under optimal conditions of both field studies (average across). The regression analysis revealed that RCI had a strong and negative relationship (P < 0.01) with Pn and Fv/Fm under high-temperature stress of both field studies (average across) in HS accessions (− 83 and − 91%), while the relationship was non-significant but negative (P > 0.05) in HT accessions (Fig. 3). The heat map of correlation coefficients indicated that RCI% had a strong and negative correlation (− 89%, P < 0.01) with Pn under high-temperature stress of the glasshouse study (Table 5).
Fig. 10.
Principle component analysis (PCA) (A, B, C, and D): monoplot and biplot correlation of 30 cotton accessions for relative cell injury (RCI), net photosynthetic rate (Pn), stomatal conductance (Gs), leaf temperature (LT), PG (pollens germination), superoxide dismutase (SOD), malondialdehyde (MDA) and seed cotton yield (SCY) under stressful conditions of glasshouse
Fig. 3.
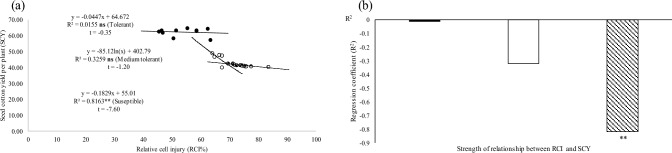
Regression analysis of relative cell injury (RCI%) of groups of tolerant (●), moderately tolerant (○), and susceptible accessions ( ) with a net photosynthetic rate, b strength of the relationship of RCI with Pn, c chlorophyll fluorescence and d the strength of the relationship of RCI with Fv/Fm under stressful conditions of the glasshouse were analyzed statistically at P < 0.01 and P < 0.05
) with a net photosynthetic rate, b strength of the relationship of RCI with Pn, c chlorophyll fluorescence and d the strength of the relationship of RCI with Fv/Fm under stressful conditions of the glasshouse were analyzed statistically at P < 0.01 and P < 0.05
Table 5.
Correlation matrix of relative cell injury, net photosynthetic rate, stomatal conductance, leaf temperature, pollens germination, malondialdehyde, superoxide dismutase and seed cotton yield under stressful conditions of glasshouse
| Variables | RCI | Pn | Gs | LT | PG | MDA | SOD | SCY |
|---|---|---|---|---|---|---|---|---|
| RCI | 1 | − 0.9594** | − 0.9302** | 0.9213** | − 0.9521** | 0.7040* | − 0.9433** | − 0.9011** |
| Pn | − 0.8994** | 1 | 0.9788** | − 0.9735** | 0.9862** | − 0.7219* | 0.9854** | 0.9827** |
| Gs | − 0.9302** | 0.9788** | 1 | − 0.9454** | 0.9672** | − 0.7500* | 0.9532** | 0.9587** |
| LT | 0.9213** | − 0.9735** | − 0.9454** | 1 | − 0.9684** | 0.8307** | − 0.9874** | − 0.9846** |
| PG | − 0.9521** | 0.9862** | 0.9672** | − 0.9684** | 1 | − 0.7529* | 0.9842** | 0.9837** |
| MDA | 0.8040** | − 0.8219** | − 0.8500** | 0.8307** | − 0.7529* | 1 | − 0.8036** | − 0.7618* |
| SOD | − 0.9433** | 0.9854** | 0.9532** | − 0.9874** | 0.9842** | − 0.8036** | 1 | 0.9919** |
| SCY | − 0.9511** | 0.9827** | 0.9587** | − 0.9846** | 0.9837** | − 0.8162** | 0.9919** | 1 |
RCI relative cell injury, Pn net photosynthetic rate, Gs stomatal conductance, LT leaf temperature, PG pollens germination, MDA malondialdehyde, SOD superoxide dismutase, SCY seed cotton yield
*Significant at 5% level of probability, **Significant at 1% level of probability
Stomatal conductance and transpiration rate
The interactive effects of heat levels (H) and genotypes (G) showed significant effects on stomatal conductance (Gs) and transpiration rate (E) under glass house (P < 0.01) and field studies (P < 0.05) (Fig. 4, Tables S5 and S6).
Fig. 4.
Impact of heat levels and genotypes of glasshouse (heat levels × genotypes P < 0.01) and field studies (heat levels × genotypes P < 0.05) on a stomatal conductance, b transpiration rate of three top heat stress tolerant (AA-703, MNH-886, VH-282) and three most heat sensitive (H-114, IR-3701, S-3) cotton genotypes at flowering. Values are the means of three replications (n = 3) ± SE and variants possessing the same letters are not statistically significant at P < 0.01 and P < 0.05)
Thirty cotton accessions were grouped as heat-tolerant (HT), moderately heat-tolerant, and heat susceptible (HS) by taking the average of their means. The HT accessions had Gs and E values above average while HS accessions had values below average (Table S7).
Among HT (1–10) and HS genotypes (16–30) (Table S7), the three HS genotypes in Fig. 4 indicated 35% and 47% lower Gs and E over the three HT genotypes (average across) under high temperature stress of both studies (average across) while the reduction in both Gs and E (average across) of HS genotypes was observed by 14% over the HT genotypes under optimal temperature of both studies (average across) The GS and E had showed highly strong and positive relation between themselves, and the HT genotypes showed higher values of GS and E in positive quadrate of PCA over the HS genotypes in negative quadrate of PCA (Fig. 10). The heat map of correlation coefficients indicates that RCI had − 93% strong and negative correlation with GS under high temperature stress of glass house study (P ˂ 0.01) (Table 5) while the regression analyses in Fig. 5 shows that the GS of HS genotypes had − 86% strong and negative relation with RCI under high temperature stress of both field of studies (average across), the HT genotypes represent the non-significant but negative relation (P ˃ 0.05) of RCI and GS under high temperature stress.
Fig. 5.
Regression analysis of relative cell injury (RCI%) of groups of tolerant (●), moderately tolerant (○), and susceptible accessions ( ) with a stomatal conductance and b the strength of the relationship of RCI with Gs under stressful conditions of a glasshouse were analyzed statistically at P < 0.01 and P < 0.05
) with a stomatal conductance and b the strength of the relationship of RCI with Gs under stressful conditions of a glasshouse were analyzed statistically at P < 0.01 and P < 0.05
Leaf physiology
The relative cell injury (RCI) and leaf temperature (LT) varied significantly for heat levels (H) and genotypes (G) under glasshouse (H × G P < 0.01) and field studies (H × G P < 0.05) (Fig. 6, Tables S8, S9). The cotton accessions were grouped as HT, MHT and HS for RCI and LT by comparing the mean of each genotype with a grand mean of thirty accessions (Table S10). The highly strong and negative relation of RCI and LT with other attributes under PCA of Fig. 10 indicates that HT genotypes had less RCI and LT over the HS genotypes, as the HT genotypes are on positive quadrate while HS genotypes along with RCI and LT are in negative quadrate of PCA (Fig. 10).
Fig. 6.
Impact of heat levels and genotypes of glass house (heat levels × genotypes P < 0.01) and field studies (heat levels × genotypes P < 0.05) on a relative cell injury and b leaf temperature of three top heat stress tolerant (AA-703, MNH-886, VH-282) and three most heat sensitive (H-114, IR-3701, S-3) cotton genotypes at flowering. Values are the means of three replications (n = 3) ± SE and variants possessing the same letters are not statistically significant at P < 0.01 and P < 0.05)
Out of 16–30 HS genotypes (Table S10), the three representative HS genotypes showed 41 and 25% more RCI and LT (average across) over the three HT genotypes (average across) under high temperatures of both field studies (average across) (Fig. 6a and b). The RCI and LT had − 92% strong and negative correlation between themselves under the heat map of correlation coefficients (r) of stressful conditions of the glasshouse study (P ˂ 0.01) (Table 5).
Seed cotton yield per plant and pollens germination/viability
The optimal and heat stress conditions of glass house (P ˂ 0.01) and field studies (P ˂ 0.05) showed significantly different responses on seed cotton yield (SCY) and pollens germination (PG) of cotton genotypes (Fig. 7, Tables S11, S12). The cotton accession having a mean value of SCY and PG above the grand mean of thirty accessions were grouped as HT (1–10) while the accessions having SCY and PG below the grand mean of thirty accessions were grouped as HS (Table S13). Similar to Table S13, the PCA in Fig. 10 grouped the genotypes with higher SCY and PG as HT in the positive quadrate while the genotypes with a lower value of SCY and PG were grouped as MHT and HS in the negative quadrate.
Fig. 7.
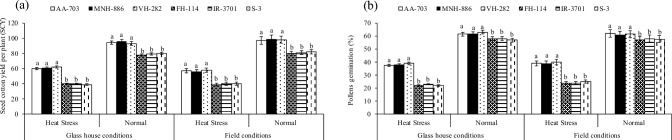
Impact of heat levels and genotypes of glass house (heat levels × genotypes P < 0.01) and field studies (heat levels × genotypes P < 0.05) on a seed cotton yield per plant and b pollens germination of three top heat stress tolerant (AA-703, MNH-886, VH-282) and three most heat sensitive (H-114, IR-3701, S-3) cotton genotypes at flowering. Values are the means of three replications (n = 3) ± SE and variants possessing the same letters are not statistically significant at P < 0.01 and P < 0.05)
Figure 7a and b show that the HS of genotypes (average across) had 34 and 41% lower SCY and PG, respectively, under high temperatures in both field studies (average across) over the three HT genotypes. The regression analysis revealed that RCI had a strong and negative relation with SCY (− 81%) of HS genotypes while HT genotypes showed non-significant but negative relation (P ˃ 0.05) of RCI with SCY under high-temperature regimes in both field studies (Fig. 8). Correlation coefficients (r) of heat map indicates RCI had − 95% correlation with SCY and PG (average across) under high-temperature stress of glass house study (P ˂ 0.01) (Table 5).
Fig. 8.
Regression analysis of relative cell injury (RCI%) of groups of tolerant (●), moderately tolerant (○), and susceptible accessions ( ) with a seed cotton yield per plant and b the strength of the relationship between RCI and SCY under stressful conditions of the glasshouse were analyzed statistically at P < 0.01 and P < 0.05
) with a seed cotton yield per plant and b the strength of the relationship between RCI and SCY under stressful conditions of the glasshouse were analyzed statistically at P < 0.01 and P < 0.05
Cell biochemistry
Heat levels (H) and genotypes (G) showed significant effects on leaf cell superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and malondialdehyde (MDA) contents under heat stress conditions of glass house (H × G P ˂ 0.01) and field studies (H × G P˂0.05) (Fig. 9 and Tables S14–S19).
Fig. 9.
Impact of heat levels and genotypes of glass house (heat levels × genotypes P < 0.01) and field studies (heat levels × genotypes P < 0.05) on the leaf a superoxide dismutase, b peroxidase, c catalase and d malondialdehyde contents of three top heat stress tolerant (AA-703, MNH-886, VH-282) and three most heat sensitive (H-114, IR-3701, S-3) cotton genotypes at flowering. Values are the means of three replications (n = 3) ± SE and variants possessing the same letters are not statistically significant at P < 0.01 and P < 0.05)
In glasshouse and field studies, heat-tolerant cotton genotypes (top 10) showed higher leaf antioxidant levels, including SOD, POD, and CAT, and lower MDA content under normal and heat conditions. Moderately heat-tolerant genotypes (11–15) and heat-susceptible genotypes (16–30) performed less well. In both field studies, heat-tolerant genotypes also showed higher antioxidant levels and lower MDA levels than medium and heat-susceptible genotypes. For example, under high-temperature stress, AA-703, MNH-886, and VH-282 produced 66, 48, and 98% more SOD, POD, and CAT, respectively, but 42% less MDA than FH-114, IR-3701, and S-3, three heat-susceptible genotypes. Additionally, the PCA indicated that HT genotypes had higher SOD, POD, and CAT but lower MDA and were placed in the positive quadrant, while MHT and HS genotypes had higher MDA but lower antioxidants and were placed in the negative quadrant of PCA (Fig. 10). The heat map of the correlation matrix revealed that SOD had a strong, positive correlation with Pn, Gs, PG, and SCY, while MDA had a strong, negative correlation with Pn, Gs, PG, and SCY (Table 5).
The bold part of heatmap in the correlation matrix indicates the strong and positive correlation of Pn, Gs, PG, SOD and SCY with each other while the italic part of heatmap shows the strong and negative correlation of RCI, LT and MDA with Pn, Gs, PG, SOD and SCY.
Principal component analysis projection for cotton genotypes
The relative effects of RCI, Pn, Gs, LT, PG, MDA, SOD, and SCY on thirty cotton genotypes were estimated using principal component analysis and classified as HT, MHT, and HS genotypes (Fig. 9).
During heat stress in cotton genotypes, the PCA loading matrix reveals a negative relation of RCI, LT, and MDA with gas exchange components, antioxidants, and seed cotton yield. The first principal component analysis (PC 1) covers 85.64% of the total variation, while the second principal component analysis (PC 2) covers 11.97% (Fig. 10). The X dots indicate HT genotypes, Y dots represent MHT genotypes, and Z dots represent HS genotypes. The better performance of heat-tolerant genotypes is due to higher Pn, Gs, PG, SOD, and SCY under high-temperature stress, while the weak performance of HS genotypes is due to higher production of RCI, LT, and MDA under high-temperature stress.
Discussion
Studies have shown that high temperatures have adverse effects on cotton crops (Kakani et al. 2005; Zhao et al. 2005; Saleem et al. 2021). Cotton plants are particularly vulnerable to temperatures above 40 °C, with flowers and pollens being the most sensitive stage (Masoomi‐Aladizgeh et al. 2021). This study aimed to evaluate heat-tolerant genotypes during flowering using leaf physiology and cell biochemical techniques. Results showed that HT genotypes had better leaf physiology, cell biochemistry, and pollens germination under normal conditions, indicating a superior defensive system even in normal environments (Mohamed and Abdel-Hamid 2013). Temperature regimes significantly affected tested materials in the field and glasshouse experiments, as evidenced by the environment versus temperature interaction (Azhar et al. 2009). Heat-tolerant accessions with a low leaf temperature and resistance to membrane leakage can be used in breeding programs to develop better heat-tolerant accessions for semi-arid areas.
HS genotypes showed a decrease in net photosynthetic rate, chlorophyll fluorescence, and stomatal conductance with each degree rise in temperature from 35 to 45 °C compared to HT genotypes. The reduction in gas exchange components in HS genotypes could be due to less production of antioxidants with more oxidative stress (Yousaf et al. 2023). On the other hand, the heat-tolerant genotypes showed a balance between antioxidants and ROS under all thermal regimes, indicating a higher production of antioxidants even under optimal environments (Zafar et al. 2021). High temperatures impair the photosynthetic capacity of cotton leaves (Pettigrew 2008), and the stomatal conductance in cotton leaves reduces when the temperature exceeds 30.1 °C (Conaty et al. 2012). HT genotypes show higher stomatal conductance, water transpiration, CO2 intake, and photosynthetic rate due to a low leaf temperature (Zafar et al. 2021). High-temperature stress also reduces the chlorophyll fluorescence in field crops due to the overproduction of electrons in thylakoid membranes (Ristic et al. 2007; Djanaguiraman et al. 2010; Sekmen et al. 2014).
According to Sekmen et al. (2014), high-temperature stress increases antioxidants in plants, but this increase is insufficient to balance the plant's defense system and reactive oxygen species (ROS). However, HT genotypes show less damage from ROS due to their higher antioxidant production. This suggests that overproduction of electrons and energy-rich compounds under heat stress can lead to oxidative bursts (Wang et al. 2018). Studies by Zahid et al. (2016) and Allakhverdiev et al. (2008) indicate that HS genotypes experience oxidative damage during high-temperature stress at flowering due to the weak efficiency of their photosynthetic machinery to utilize electrons.
In the present study, HS genotypes showed a 3 and 4% decrease in seed cotton yield and pollen germination, respectively, for each degree rise in temperature from 35 to 45 °C, whereas HT accessions demonstrated better membrane stability, gas exchange components, photosynthetic efficiency, and a balance between antioxidants and ROS, leading to higher pollen germination and seed cotton yield (Azhar et al. 2009). High temperatures can affect pollen germination by impairing enzyme function and reducing the duration of pollen receptivity of the stigma, thereby shortening the time for successful pollination (Snider et al. 2009). Pollen germination in cotton is also reported to decrease by 40% at 39 °C due to reduced sugar availability to the developing pollen (Burke 2011). Additionally, high temperatures inhibit photosynthesis, which is linked to low cotton yield (Najeeb et al. 2017).
These findings support the hypothesis that screening cotton genotypes based on leaf physiology, cell biochemistry, and pollen germination can be a better technique to evaluate heat-tolerant genotypes, and breeders can use these techniques to introduce sustainable cotton genotypes under predicted environments.
The second objective of this study was to investigate the relationship between RCI, LT, and MDA with Pn, Gs, E, PG, and SCY in HT and HS genotypes under normal and stressful conditions. The results showed a significant negative correlation between RCI, LT, and MDA with Pn, GS, E, Fv/Fm, SOD, PG, and SCY under stress. These findings were consistent with previous studies (Blum 1998; Hameed and Ali 2016), where RCI, LT, and MDA were strongly and negatively associated with other attributes, particularly in heat-susceptible genotypes. Principal Component Analysis (PCA) was used to describe the relation and variation among the variables of accessions and cluster them into various groups based on genetic diversity. The PCA results indicated that Pn, Gs, PG, SOD, and SCY had a strong positive association with HT accessions, while RCI, LT, and MDA had a negative association, particularly with HS genotypes. These findings suggest that physiological, biochemical, and pollen germination traits could be useful indicators of accession identification under stressful environments. PCA is a valuable tool for evaluating variety in future environments.
Conclusion
We conclude that high temperature (45 °C) significantly damaged leaf physiological, cell biochemistry and pollen germination functions due to high membrane leakage. The relative cellular injury was negatively associated with SCY, Fv/Fm, Pn, Gs, E, PG, SOD, POD and CAT under optimal thermal regimes, but the relationship was strong under high-temperature regimes. A significant accession versus environment interaction for cotton yield under hot environments indicated the presence of heat tolerance in cotton germplasm, which can be exploited through breeding programs. The PCA grouped the accessions into heat-tolerant, moderately heat-tolerant, and heat susceptible accessions based on leaf physiological, cell biochemical and pollens germination trials and extracted good information on genetic diversity under heat-stress environments. This study indicates that cotton accessions with superior chlorophyll fluorescence, gas exchange, pollen germination, and antioxidants with lower malondialdehyde contents could sustain lipid membrane stability and lint yield in hot environments. Thus, leaf physiological, cell biochemical and pollens germination traits could be used as a good marker for selecting heat-tolerant germplasm. Further studies are needed to test these methods, along with the presence of candidate genes in heat tolerance on large cotton accessions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The Higher Education Commission of Pakistan (HEC) and Dongguk University, South Korea, financially supported this report. The writers are very grateful for providing technical support University of Agriculture Faisalabad, Pakistan, and Dongguk University, South Korea. Dongguk University, South Korea, and Higher Education Commission of Pakistan (HEC), financially supported this report. The writers are very grateful for providing technical support of Dongguk University, South Korea, and University of Agriculture Faisalabad, Pakistan.
Abbreviations
- CMT
Cell membrane thermostability
- Fv/Fm
Chlorophyll fluorescence
- EC
Electrical conductivity
- Pn
Net photosynthetic rate
- RCI
Relative cell injury
- GS
Stomatal conductance
- SCY
Seed cotton yield
- E
Transpiration rate
- PG
Pollens germination
- SOD
Superoxide dismutase
- POD
Peroxidase
- CAT
Catalase
- MDA
Malondialdehyde
Author contributions
MK, MFS, MS, and NU planned and designed the research protocol; MS carried out the experiment and collected and analyzed samples. MK, and MKM, supervised the study and organized and analyzed data. MK, NU, MFS, BC, and AA technically supported the research and wrote the manuscript. MK acquires funding.
Funding
MK for Dongguk university support fund 2022–24.
Declarations
Conflict of interest
All the authors declare no conflicts of interest regarding this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ababaei B, Chenu K. Heat shocks increasingly impede grain filling but have little effect on grain setting across the Australian wheatbelt. J Agric Meteorol. 2020;284:107889. doi: 10.1016/j.agrformet.2019.107889. [DOI] [Google Scholar]
- Abro S, Rajput MT, Khan MA, Sial MA, Tahir SS. Screening of cotton (Gossypium hirsutum L.) genotypes for heat tolerance. Pak J Bot. 2015;47:2085–2091. [Google Scholar]
- Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P. Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res. 2008;98:541–550. doi: 10.1007/s11120-008-9331-0. [DOI] [PubMed] [Google Scholar]
- Azhar F, Ali Z, Akhtar M, Khan A, Trethowan R. Genetic variability of heat tolerance, and its effect on yield and fibre quality traits in upland cotton (Gossypium hirsutum L.) Plant Breed. 2009;128:356–362. doi: 10.1111/j.1439-0523.2008.01574.x. [DOI] [Google Scholar]
- Bel G, Teixidó JJ. The political economy of the Paris agreement: income inequality and climate policy. J Clean Prod. 2020;258:121002. doi: 10.1016/j.jclepro.2020.121002. [DOI] [Google Scholar]
- Bibi A, Oosterhuis D, Gonias E. Photosynthesis, quantum yield of photosystem II and membrane leakage as affected by high temperatures in cotton genotypes. J Cotton Sci. 2008;12:150–159. [Google Scholar]
- Blum A. Improving wheat grain filling under stress by stem reserve mobilisation. Euphytica. 1998;100:77–83. doi: 10.1023/A:1018303922482. [DOI] [Google Scholar]
- Brown RS, Oosterhuis DM (2004) High daytime temperature stress effects on the physiology of modern versus obsolete cotton cultivars. In: Summaries of cotton research in 1, pp 63–67
- Burke JJ, Wanjura DF (2010) Plant responses to temperature extremes. In: Physiology of cotton, pp 123–128
- Burke JJ (2011) Cotton flowers: pollen and petal humidity sensitivities determine reproductive competitiveness in diverse environments. In: Stress physiology in cotton, pp 25
- Cakmak I, Horst WJ. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiol Plant. 1991;83:463–468. doi: 10.1111/j.1399-3054.1991.tb00121.x. [DOI] [Google Scholar]
- Chastain DR, Snider JL, Choinski JS, Collins GD, Perry CD, Whitaker J, Grey TL, Sorensen RB, van Iersel M, Byrd SA. Leaf ontogeny strongly influences photosynthetic tolerance to drought and high temperature in Gossypium hirsutum. J Plant Physiol. 2016;199:18–28. doi: 10.1016/j.jplph.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Conaty W, Burke J, Mahan J, Neilsen J, Sutton B. Determining the optimum plant temperature of cotton physiology and yield to improve plant-based irrigation scheduling. Crop Sci. 2012;52:1828–1836. doi: 10.2135/cropsci2011.11.0581. [DOI] [Google Scholar]
- Cottee N, Tan D, Bange M, Cothren J, Campbell L. Multi-level determination of heat tolerance in cotton (Gossypium hirsutum L.) under field conditions. Crop Sci. 2010;50:2553–2564. doi: 10.2135/cropsci2010.03.0182. [DOI] [Google Scholar]
- Demİrel U, Çopur O, Gür A. Early-stage screening for heat tolerance in cotton. Plant Breed. 2016;135:80–89. doi: 10.1111/pbr.12333. [DOI] [Google Scholar]
- Djanaguiraman M, Sheeba JA, Devi DD, Bangarusamy U, Prasad P. Nitrophenolates spray can alter boll abscission rate in cotton through enhanced peroxidase activity and increased ascorbate and phenolics levels. J Plant Physiol. 2010;167:1–9. doi: 10.1016/j.jplph.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutases: I. Occurrence in higher plants. J Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez KA, Gomez AA. Statistical procedures for agricultural research. Wiley; 1984. pp. 67–70. [Google Scholar]
- Hameed S, Ali MK. Exogenous application of salicylic acid: inducing thermotolerance in cotton (Gossypium hirsutum L.) seedlings. Int J Agric Res. 2016;5:9–18. [Google Scholar]
- Jarwar AH, Wang X, Iqbal MS, Sarfraz Z, Wang L, Ma Q, Shuli FJPJB. Genetic divergence on the basis of principal component, correlation and cluster analysis of yield and quality traits in cotton cultivars. Pak J Bot. 2019;51:1143–1148. doi: 10.30848/PJB2019-3(38). [DOI] [Google Scholar]
- Kakani V, Reddy K, Koti S, Wallace T, Prasad P, Reddy V, Zhao D. Differences in in vitro pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Ann Bot. 2005;96:59–67. doi: 10.1093/aob/mci149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karademir E, Karademir Ç, Sevilmis U, Basal HJFEB. Correlations between canopy temperature, chlorophyll content and yield in heat-tolerant cotton (Gossypium hirsutum L.) genotypes. Fresenius Environ Bull. 2018;27:5230–5237. [Google Scholar]
- Li Z, Wang X, Zhang Y, Zhang G, Wu L, Chi J, Ma Z. Assessment of genetic diversity in glandless cotton germplasm resources by using agronomic traits and molecular markers. Front Agric China. 2008;2:245–252. doi: 10.1007/s11703-008-0063-x. [DOI] [Google Scholar]
- Liu M, Sun J, Sun Y, Bock C, Chen Q. Thickness-dependent mechanical properties of polydimethylsiloxane membranes. J Micromech Microeng. 2009;19:035028–035032. doi: 10.1088/0960-1317/19/3/035028. [DOI] [Google Scholar]
- Loka DA, Oosterhuis DM, Baxevanos D, Noulas C, Hu WJPP, Biochemistry, Single and combined effects of heat and water stress and recovery on cotton (Gossypium hirsutum L.) leaf physiology and sucrose metabolism. Plant Physiol Biochem. 2020;148:166–179. doi: 10.1016/j.plaphy.2020.01.015. [DOI] [PubMed] [Google Scholar]
- Mantri N, Patade V, Pang E (2014) Recent advances in rapid and sensitive screening for abiotic stress tolerance. In: Improvement of crops in the era of climatic changes, pp 37–47
- Masoomi-Aladizgeh F, Najeeb U, Hamzelou S, Pascovici D, Amirkhani A, Tan DK, Mirzaei M, Haynes PA, Atwell BJ. Pollen development in cotton (Gossypium hirsutum) is highly sensitive to heat exposure during the tetrad stage. Plant Cell Environ. 2021;44:2150–2166. doi: 10.1111/pce.13908. [DOI] [PubMed] [Google Scholar]
- Min L, Li Y, Hu Q, Zhu L, Gao W, Wu Y, Ding Y, Liu S, Yang X, Zhang X. Sugar and auxin signaling pathways respond to high-temperature stress during anther development as revealed by transcript profiling analysis in cotton. Plant Physiol. 2014;164:1293–1308. doi: 10.1104/pp.113.232314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed H, Abdel-Hamid A. Molecular and biochemical studies for heat tolerance on four cotton genotypes. Rom Biotechnol Lett. 2013;18:8823–8831. [Google Scholar]
- Najeeb U, Jilani G, Ali S, Sarwar M, Xu L, Zhou W. Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J Hazard Mater. 2011;186:565–574. doi: 10.1016/j.jhazmat.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Najeeb U, Sarwar M, Atwell BJ, Bange MP, Tan DK. Endogenous ethylene concentration is not a major determinant of fruit abscission in heat-stressed cotton (Gossypium hirsutum L.) Front Plant Sci. 2017;8:1615. doi: 10.3389/fpls.2017.01615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveed S, Aslam M, Maqbool M, Bano S, Zaman Q, Ahmad R. Physiology of high temperature stress tolerance at reproductive stages in maize. J Anim Plant Sci. 2014;24:1141–1145. [Google Scholar]
- Pettigrew WT. Improved yield potential with an early planting cotton production system. J Agron. 2002;94:997–1003. doi: 10.2134/agronj2002.9970. [DOI] [Google Scholar]
- Pettigrew W. The effect of higher temperatures on cotton lint yield production and fiber quality. Crop Sci. 2008;48:278–285. doi: 10.2135/cropsci2007.05.0261. [DOI] [Google Scholar]
- Prasad PV, Pisipati S, Ristic Z, Bukovnik U, Fritz A. Impact of nighttime temperature on physiology and growth of spring wheat. Crop Sci. 2008;48:2372–2380. doi: 10.2135/cropsci2007.12.0717. [DOI] [Google Scholar]
- Qiaoling W, Zhe L. Principal component analysis of F2 individual selection in upland cotton (Gossypium hirsutum L.) J Henan Inst Sci Technol. 2011;5:54–64. [Google Scholar]
- Reddy KR, Davidonis GH, Johnson AS, Vinyard BT. Temperature regime and carbon dioxide enrichment alter cotton boll development and fiber properties. J Agron. 1999;91:851–858. doi: 10.2134/agronj1999.915851x. [DOI] [Google Scholar]
- Rehman SU, Abid MA, Bilal M, Ashraf J, Liaqat S, Ahmed RI, Qanmber G. Genotype by trait analysis and estimates of heritability of wheat (Triticum aestivum L.) under drought and control conditions. Basic Res J Agric Sci Rev. 2015;4:127–134. [Google Scholar]
- Ristic Z, Bukovnik U, Prasad PV. Correlation between heat stability of thylakoid membranes and loss of chlorophyll in winter wheat under heat stress. Crop Sci. 2007;47:2067–2073. doi: 10.2135/cropsci2006.10.0674. [DOI] [Google Scholar]
- Saleem MA, Malik W, Qayyum A, Ul-Allah S, Ahmad MQ, Afzal H, Amjid MW, Ateeq MF, Zia ZUJMBR. Impact of heat stress responsive factors on growth and physiology of cotton (Gossypium hirsutum L.) Mol Biol Rep. 2021;48:1069–1079. doi: 10.1007/s11033-021-06217-z. [DOI] [PubMed] [Google Scholar]
- Salman M, Zia Zu, Rana IA, Maqsood RH, Ahmad S, Bakhsh A, Azhar MT. Genetic effects conferring heat tolerance in upland cotton (Gossypium hirsutum L.) J Cotton Res. 2019;2:1–8. doi: 10.1186/s42397-019-0025-2. [DOI] [Google Scholar]
- Sarwar M, Saleem M, Najeeb U, Shakeel A, Ali S, Bilal M. Hydrogen peroxide reduces heat-induced yield losses in cotton (Gossypium hirsutum L.) by protecting cellular membrane damage. J Agron Crop Sci. 2017;203:429–441. doi: 10.1111/jac.12203. [DOI] [Google Scholar]
- Sarwar M, Saleem MF, Ullah N, Rizwan M, Ali S, Shahid MR, Alamri SA, Alyemeni MN, Ahmad P. Exogenously applied growth regulators protect the cotton crop from heat-induced injury by modulating plant defense mechanism. Sci Rep. 2018;8:1–15. doi: 10.1038/s41598-018-35420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar M, Saleem MF, Ullah N, Ali S, Rizwan M, Shahid MR, Alyemeni MN, Alamri SA, Ahmad P. Role of mineral nutrition in alleviation of heat stress in cotton plants grown in glasshouse and field conditions. Sci Rep. 2019;9:1–17. doi: 10.1038/s41598-019-49404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenker W, Roberts MJ. Nonlinear temperature effects indicate severe damages to US crop yields under climate change. Proc Natl Acad Sci. 2009;106:15594–15598. doi: 10.1073/pnas.0906865106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekmen AH, Ozgur R, Uzilday B, Turkan IJE, Botany E. Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ Exp Bot. 2014;99:141–149. doi: 10.1016/j.envexpbot.2013.11.010. [DOI] [Google Scholar]
- Singh K, Wijewardana C, Gajanayake B, Lokhande S, Wallace T, Jones D, Reddy KR. Genotypic variability among cotton cultivars for heat and drought tolerance using reproductive and physiological traits. Euphytica. 2018;214:1–22. doi: 10.1007/s10681-018-2135-1. [DOI] [Google Scholar]
- Snider JL, Oosterhuis DM, Skulman BW, Kawakami EM. Heat stress-induced limitations to reproductive success in Gossypium hirsutum. Physiol Plant. 2009;137:125–138. doi: 10.1111/j.1399-3054.2009.01266.x. [DOI] [PubMed] [Google Scholar]
- Steel RGD, Torrie JH (1960) Principles and procedures of statistics. In: Principles and procedures of statistics, pp 481
- Stocker T. Climate change 2013: the physical science basis: Working Group I contribution to the Fifth assessment report of the Intergovernmental Panel on Climate Change. England: Cambridge University Press; 2014. [Google Scholar]
- Sullivan CY. Mechanism of heat and drought resistance in grain sorghum and methods of measurement. Sorghum in the Seventies. JPN J Crop Sci. 1972;43:385–390. [Google Scholar]
- Taylor RM. Germination of cotton (Gossypium hirsutum L.) Pollen on an artificial medium 1. Crop Sci. 1972;12:243–244. doi: 10.2135/cropsci1972.0011183X001200020030x. [DOI] [Google Scholar]
- Ullah A, Shakeel A, Malik T, Saleem M. Assessment of drought tolerance in some cotton genotypes based on drought tolerance indices. J Anim Plant Sci. 2019;29:998–1009. [Google Scholar]
- Ullah Q, Ahmad MZ, Ullah K, Sayal O, Jamil A, Mohibullah M, Ahmad B (2020) Investigation of cotton germplasm for genetic divergence regarding yield related trait using principal component analysis, pp 1–12
- Wang X, Cai J, Jiang D, Liu F, Dai T, Cao W. Pre-anthesis high-temperature acclimation alleviates damage to the flag leaf caused by post-anthesis heat stress in wheat. J Plant Physiol. 2011;168:585–593. doi: 10.1016/j.jplph.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Wang QL, Chen JH, He NY, Guo FQ. Metabolic reprogramming in chloroplasts under heat stress in plants. Int J Mol Sci. 2018;19:849. doi: 10.3390/ijms19030849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousaf MI, Hussain Q, Alwahibi MS, Aslam MZ, Khalid MZ, Hussain S, Zafar A, Shah SAS, Abbasi AM, Mehboob A. Impact of heat stress on agro-morphological, physio-chemical and fiber related parameters in upland cotton (Gossypium hirsutum L.) genotypes. J King Saud Univ Sci. 2023;35:102379. doi: 10.1016/j.jksus.2022.102379. [DOI] [Google Scholar]
- Zafar MM, Manan A, Razzaq A, Zulfqar M, Saeed A, Kashif M, Khan AI, Sarfraz Z, Mo H, Iqbal MS. Exploiting agronomic and biochemical traits to develop heat resilient cotton cultivars under climate change scenarios. Agronomy. 2021;11:1885–1899. doi: 10.3390/agronomy11091885. [DOI] [Google Scholar]
- Zahid KR, Ali F, Shah F, Younas M, Shah T, Shahwar D, Hassan W, Ahmad Z, Qi C, Lu Y. Response and tolerance mechanism of cotton Gossypium hirsutum L. to elevated temperature stress: a review. Front Plant Sci. 2016;7:937–950. doi: 10.3389/fpls.2016.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Reddy KR, Kakani VG, Koti S, Gao W. Physiological causes of cotton fruit abscission under conditions of high temperature and enhanced ultraviolet-B radiation. Physiol Plant. 2005;124:189–199. doi: 10.1111/j.1399-3054.2005.00491.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.