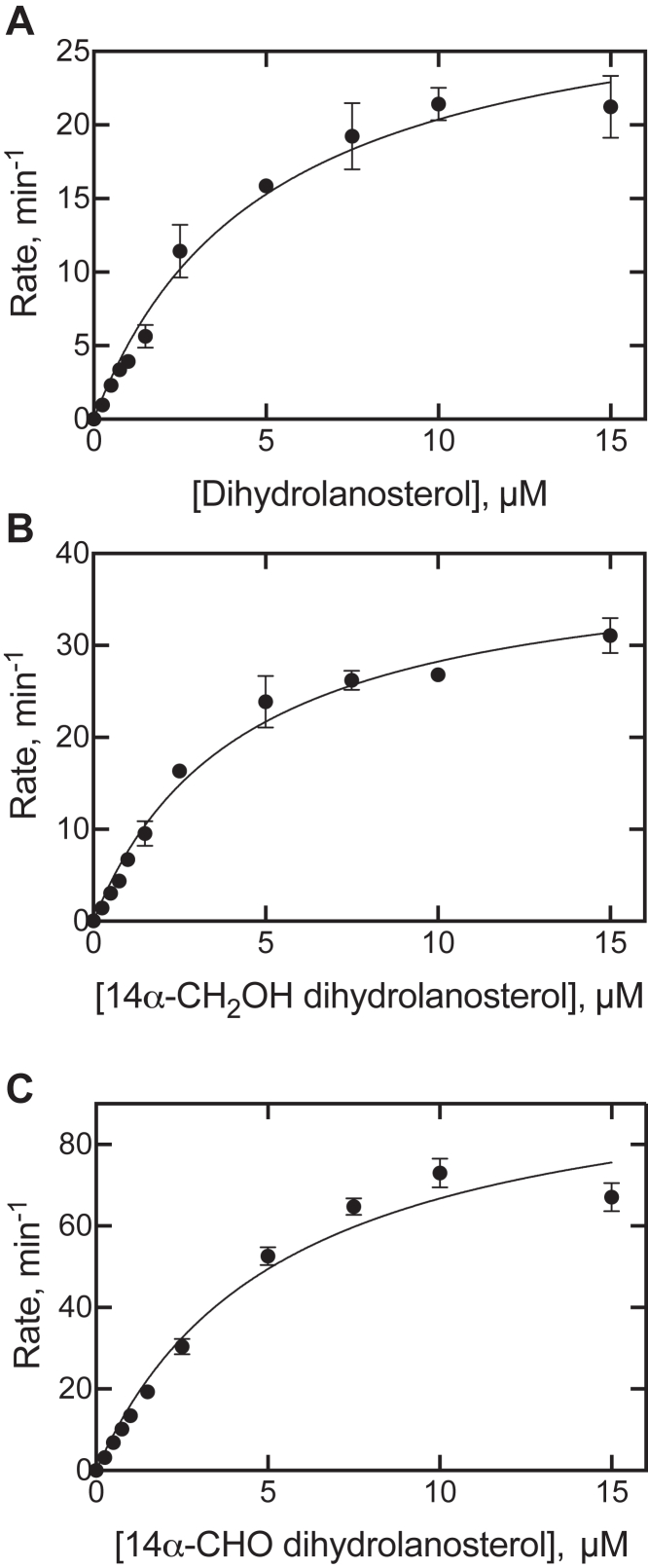
Figure 7.
Steady-state kinetics of oxidation of dihydrolanosterol and its 14α-CH2OH and 14α-CHO derivatives to dihydro FF-MAS. The individual substrates were incubated with a reconstituted P450 51A1 system, and the product dihydro FF-MAS was extracted and analyzed by UPLC-UV. A, dihydrolanosterol: kcat 0.50 ± 0.03 s−1, Km 5.0 ± 0.6 μM; B, 14α-CH2OH dihydrolanosterol: kcat 0.67 ± 0.03 s−1, Km 4.3 ± 0.4 μM; C, 14α-CHO dihydrolanosterol: kcat 1.7 ± 0.1 s−1, Km 5.4 ± 0.7 μM. Rates are presented on the y-axis as nmol product (dihydro FF-MAS) formed min−1(nmol P450)−1. Incubations were run in triplicate, and the means (±SD) were calculated and plotted, The linear regression fits include the Prism error estimates for internal fitting (SE). The estimated parameters were not corrected using a quadratic equation, due to the high Km values. FF-MAS, follicular fluid meiosis-activating sterol ((4β,5α)-4,4-dimethyl-cholesta-8,14,24-trien-3-ol); UPLC, ultra-performance liquid chromatography.