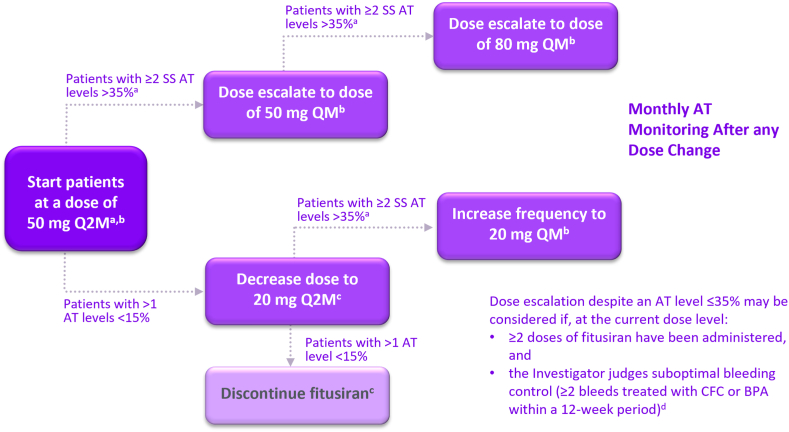
Figure 5.
Fitusiran revised dose and dose regimen, targeting an antithrombin range from 15% to 35% [62]. The revised dose and dose regimen was introduced as of December 2020. BPA, bypassing agent; CFC, clotting factor concentrate; Q2M, every other month; SS, steady state. Source: Figure adapted with permission from Pipe et al. [62].
aParticipants are eligible for dose escalation if >4 doses of fitusiran have been administered at the current dose level, and they experienced >2 predose antithrombin (AT) activity levels >35% (as per central laboratory) after their second dose at the current dose, and fitusiran administration and AT activity assessments occurred as per schedule at the current dose level.
bParticipants previously escalated to a dose of 20 mg every month (QM), 50 mg QM or 80 mg QM due to AT >35% who experience >1 AT activity level <15% within a 12 month period must either permanently discontinue fitusiran prophylaxis, or in consultation with the Study Medical Manager may have the option to be de-escalated to their prior dose level.
cStart of dosing after de-escalation from higher dose to occur only after centrally measured AT levels ≥22%. Participants receiving fitusiran at a dose of 20 mg every other month who experience ≥1 AT activity level <15% (as per central laboratory) within a 12-month period must permanently discontinue fitusiran treatment.
dParticipants with QM dosing bleeding episodes during the first 8 weeks at the current dose level or every other month dosing bleeding episodes during the first 12 weeks at the current dose level will not be considered for this judgment.