Abstract

In nature, minerals record various origins and information for geology and geobiochemistry. Here, we investigated the origin of organic matter and growth mechanism of quartz with oil inclusion revealing fluorescence under short ultraviolet (UV) light, obtained from the clay vein at Shimanto-cho, Kochi, Shikoku Island, Japan. Geological investigation indicated that the oil–quartz was formed in hydrothermal metamorphic veins found in the late Cretaceous interbedded sandstone and mudstone. The obtained oil–quartz crystals are mostly double-terminated. Micro-X-ray computed tomography (microCT) indicated that oil–quartz crystals have various veins originating as skeleton structures along the quartz crystal {111} and {1–11} faces. Spectroscopic and chromatographic studies indicated that aromatic ester and tetraterpene (lycopene) molecules, which revealed fluorescence, were detected. Large molecular weight sterol molecules, such as C40, were also detected in the vein of oil–quartz. This investigation indicated that organic inclusions in mineral crystals would form with ancient microorganism culture environments.
1. Introduction
Unlike in industrial processes and laboratory materials, almost all natural minerals contain various solid–liquid–gas phases as inclusions and additives.1−3 These inclusions are occasionally regarded as unnecessary objects, but they also play precious roles for such minerals like the star effect for sapphire, phantom quartz, and alexandrite.4−7 The inclusions have recorded the conditions and processes of the geological formation and become the key to the discovery for new materials such as lasers, optical devices, semiconductors, and electronics for sustainable chemistry and natural resources.
Organic material-related structures in various scales of geological settings have attracted attention for not only mineralogy, geology, and geochemistry but also materials sciences, bioceramics, medical equipment, and green chemistry. The silica clathrate compound and layered materials, typified zeolite and mica, were already widely applied as ionic exchange materials and biomedical materials because of their biocompatibility and low environmental loading.8−11 Moreover, recently, organic molecule substitution and hybridization to silica-based minerals found in various geological scales have attracted attention for new aspects of mineralogy, igneous petrology, and astrobiology for chemical evolution, origin of life, and electrocatalytic effects,12−15 for example, “Hayabusa2 artificial satellite project.” The relationships of organic molecules and inorganic minerals were considered the key to the origin of life and Earth.16
Previous studies have reported that the typical silicate mineral quartz (SiO2) was sometimes found with organic matters in its crystals as negative crystals and/or surface coating.17−20 Suchy et al.21 discussed the formation sequences of organic inclusions in quartz obtained from the west region of the Czech Republic, analyzing by mass spectroscopic methods. However, the relationships between the states of organic inclusions in quartz and surrounding rocks were still unclear.
In this study, we focused on fluorescence quartz (oil–quartz) found from a sedimentary rock of southwest region, Shikoku Island, Southwest, Japan. Results showed that the origin of fluorescence regions in oil–quartz is attributed to its geological setting.
2. Results and Discussion
2.1. Geological Setting: Regional Geology
The regional stratigraphy and geology of the study area, Shimanto region, have been summarized by Katto et al.22 The collection site is located in the Nonogawa formation, Shimanto supergroup, developed at the southwest region of Shikoku Island, Japan (Figure 1a).23−25 The Shimanto belt was categorized as a typical accretionary prism at the subduction zone of the Philippine Sea Plate sneaking into the Eurasian Plate. Radiolarian age analysis indicated that the sedimentary age of the Nonogawa formation was later Santonian to former Campanian of the Cretaceous period.26 The Nonogawa formation primarily comprised alternate sandstone and shale layers of 10 m thickness with small amounts of chert and acidic tuff (Figure 1b).27
Figure 1.
(a) Location of Shimanto area, Kochi prefecture, Shikoku Island, Japan. (b) Geological mapping of the survey area in this study. Modified from the geological map Kubokawa, Geological Survey of Japan, 193323 with reprint permitted.
The lens-like clay veins probably formed via hydrothermal alteration are observed in the interbedded sandstone and shale. The width of the lens-like clay veins varies from several cm to m in thickness (Figure 2a). Oil–quartz crystals were found in the clay veins (Figure 2b). All rock and quartz samples were obtained from an outcrop of a stratum located at Minenoue, Shimanto-cho, Kochi prefecture, Japan; 33.169029 north, 133.139936 east. All obtained rock samples for further evaluations were trimmed to remove the surface-weathered part.
Figure 2.
(a) Photograph of the outcrop of quartz veins obtained from oil–quartz crystals. (b) Photograph of the quartz veins and paragenesis of oil–quartz crystals in veins. (c) Schematic image of the structure of quartz veins and paragenesis of oil–quartz crystals.
2.2. Occurrence of Oil–Quartz Paragenesis and Surrounding Rock Conditions
The quartz crystals were obtained from yellowish colored clay veins with sandstone. Most quartz crystals were double-terminated, and the direction of the c-axis is parallel to the strike of clay veins. Sometimes, the parallel intergrowth of quartz crystals was observed. The length of the c-direction varies from several cm to 10 cm.
The color of the adjacent sandstone was a bit dark compared to that of the normal sandstone in the outcrop. In this study, therefore, we divided rock samples into three parts based on the relationships of quartz veins: mother rock, transition zone, and clay vein (Figure 2c).
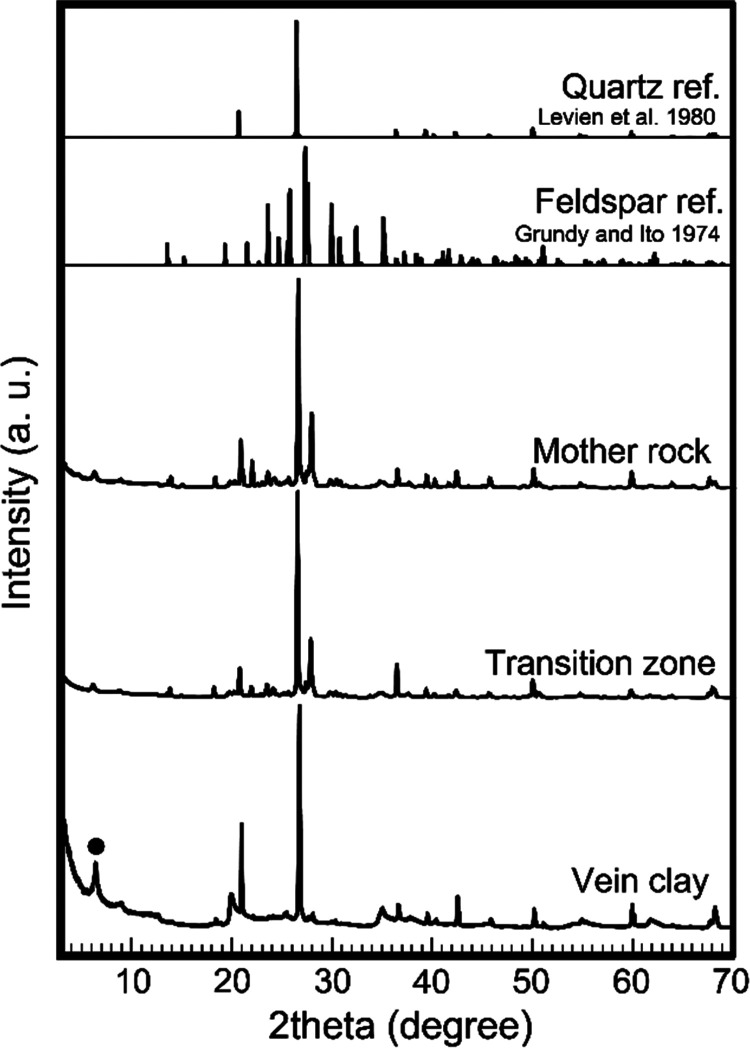
Figure 3 shows the thin sections of each zone of rock core samples. In the cases of the parent rock and transition zone, a bit rounded colored minerals and colorless minerals identified as quartz and feldspar were commonly observed, respectively. They were approximately several 100 μm in size. Many brownish organic particles around several 10 μm in sizes were also observed in quartz and feldspar crystals. In the case of the transition zone, the distribution and structure of minerals were mostly the same as those of the parent rocks, but a few chlorite particles were also observed. Meanwhile, in the clay vein, a brownish membrane structure surrounding quartz crystals was observed, instead of brownish organic particles. In addition, few feldspar particles were observed. This tendency of the mineral component in each rocks was estimated by bulk X-ray diffraction (XRD) measurement. Figure 4 shows the X-ray diffraction (XRD) patterns of each zone of rock core samples. Although obviously strong peaks identified as feldspar were detected in the parent rock and transition zone, no significant peaks of feldspar were observed in the clay vein, but several clay minerals such as montmorillonite were identified. The fine structures of clay minerals in the clay vein were observed.
Figure 3.

Thin section of the typical structure of rock cores. (a, b) Parent rock. (c, d) Transition zone. (e, f) Vein clay. Qt: quartz. Yellow broken line area: the organic particle-aggregated areas. Blue broken line: the unformed organic particle-aggregated areas.
Figure 4.
XRD patterns of rock core samples with reference quartz50 and feldspar51 patterns with {hkl} indexes for facilitating comparison. ●: montmorillonite.52
Figure 5 shows the SEM micrographs of the clay vein. The clay vein comprises scaly materials with typical clay minerals and few fiber-like particles around several 100 nm in size.
Figure 5.

SEM micrographs of the vein clay. (a) Low magnification. (b) High magnification.
The bulk compositions of inorganic and organic compounds in each samples were measured. Table 1 shows the bulk compositions of each rock core sample measured via X-ray fluorescence (XRF) spectroscopy. The clay vein contained lower K and higher Al and Fe compositions than those of the parent rock and transition zone, which indicate that the alteration of the hydrothermal solution into the parent rock caused the clay vein formation. In addition, we measured total carbon contents and organic carbon contents of samples. Figure 6 shows the total carbon contents and organic carbon contents of samples. The carbon content, particularly the organic carbon content of the clay vein, was approximately 10 times higher than those of the parent rock and transition zone.
Table 1. XRF Analysis Results of Bulk Chemical Compositions of Rock Core Samplesa.
| atom % | mother rock | transition zone | vein clay |
|---|---|---|---|
| SiO2 | 64.38 ± 0.35 | 62.16 ± 0.05 | 56.26 ± 0.30 |
| Al2O3 | 18.53 ± 0.16 | 19.62 ± 0.10 | 23.09 ± 0.09 |
| K2O | 7.39 ± 0.08 | 8.61 ± 0.13 | 4.82 ± 0.07 |
| Fe2O3 | 6.71 ± 0.20 | 6.09 ± 0.16 | 13.31 ± 0.16 |
| TiO2 | 1.10 ± 0.04 | 1.41 ± 0.03 | 1.22 ± 0.02 |
| Na2O | 0.60 ± 0.06 | 0.34 ± 0.11 | 0.00 ± 0.00 |
| P2O5 | 0.30 ± 0.06 | 0.38 ± 0.06 | 0.00 ± 0.00 |
| MnO | 0.37 ± 0.01 | 0.77 ± 0.03 | 0.35 ± 0.01 |
| MgO | 0.28 ± 0.00 | 0.21 ± 0.02 | 0.59 ± 0.02 |
| SO3 | 0.16 ± 0.01 | 0.20 ± 0.01 | 0.13 ± 0.01 |
| V2O5 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.09 ± 0.01 |
| ZrO2 | 0.03 ± 0.00 | 0.04 ± 0.01 | 0.05 ± 0.00 |
| ZnO | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.04 ± 0.00 |
| Rb2O | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| SrO | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| Ir2O3 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Cr2O3 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 |
| Y2O3 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| total | 100.00 | 100.00 | 100.00 |
The final ratios of samples were modified for total = 100.0%.
Figure 6.
Total carbon (black bar) and organic carbon (white bar) content of rock core samples.
2.3. Analysis of the Oil–Quartz Crystal Sample
Figure 7 shows the representative photograph of oil–quartz crystals. They were mostly obtained as double-terminated crystals. Under UV (235 nm) light, blue- and/or orange-colored luminescence domains were observed in oil–quartz crystals. Although the domains around several 10 μm in size (smaller distributions) were particle-like, larger luminescence domains had normally lineal or membranous shapes. In addition, the sizes of lineally or membranous shaped domains were consistent with the sizes of parent quartz crystals. The luminescence domains in the voids of quartz crystals were not always coincident with the shapes of voids of quartz. Noteworthily, the bright intensities of outer parts of luminescence domains were higher than those of inner parts.
Figure 7.

Photographs of representative oil–quartz crystals. (a) Normal light. (b) UV light. (c) Magnified image of the void under normal light. (d) Magnified image of the void under UV light. (e) Relationship of bright intensity of the luminescence area and void structure of (c).
The morphology and surface structure of quartz crystals were further evaluated. Most quartz crystals exhibited a smooth surface (Figure 7). Some of the obtained quartz crystals also exhibited typical skeleton-shaped crystals. Figure 8 shows the surface structures of typical skeleton-shaped crystals of quartz. Both {1–11} and {111} faces exhibited a typical center-recessed structure and developed a rim structure. The fine surface structures of the quartz crystals were observed by differential interference contrast (DIC) microscopy. The surface structure of the rim areas was essentially smooth; however, numerous small peaks and steps were also present. Furthermore, in the central part of the crystal surface, numerous steps of several μm in height were observed.
Figure 8.

Photographs of representative oil–quartz skeleton crystals. (a) Whole image. (b) Magnified image of the top view. (c) DIC image of the rim structure of the skeleton crystal {1–11}. (d) DIC image of the center structure of the skeleton crystal {1–11}.
MicroCT analysis of the samples indicated the fine three-dimensional (3D) structure of samples. Figure 9 shows the microCT images of representative oil–quartz crystals with photographs under normal and UV light. The 3D shapes of voids were located along the quartz {111} and {1–11} faces as bowl-type ones. The bottom of the void revealed a flat shape; on the other hand, the outer face of the void was curved. Combined with UV irradiation observation, luminescence regions were located at the bottom and edge parts of void structures. In addition, some void structures were fused to each other. These void structures illustrate typical skeleton crystal structures, which are formed at a rapid growth rate. Thus, considering the morphology of oil–quartz, i.e., double-terminated and skeleton crystals, oil–quartz crystals were homogeneously nucleated in a growth field of clay veins.
Figure 9.

MicroCT image of the representative oil–quartz crystal. (a, b) Photograph of the oil–quartz crystal for facilitating comparison. (c) Reconstruct image of the whole structure. (d) XY-slice. (e) Reconstruct slice of XZ. (f) YZ-slice.
The oil extracted from the voids of oil–quartz crystals was spectroscopically analyzed (Figure 10). In the UV region, two important irradiation bands were observed. Furthermore, in the visible region, two irradiation bands at 412 and 565 nm exhibited purple and green color luminescence, respectively.
Figure 10.
Fluorescence spectrum of the extracted oil sample from oil–quartz crystals. (a) Whole wavelength. (b) Visible region.
The CHN content of the extracted oil from oil crystals was 70.59, 11.25, and 1.07 atom %, respectively.
The oil samples were also extracted from rock core samples and oil–quartz crystals for estimating the origin of organic matters in oil–quartz crystals. Figure 11 shows the FTIR spectra of the extracted oil samples. All extracted oil samples revealed C–H, C–O, and C=O bonding of fatty acid and aromatic molecules along with the alkyl chain bonding (C–C). Furthermore, amido bonding was also slightly detected in the oil–quartz crystals.
Figure 11.
FT-IR spectra of extracted oil samples from the rock core and oil–quartz crystal samples. (a) Wide range. (b) Low wavenumber. (c) Water adsorption.
Gas chromatography–mass spectrometry (GC-MS) analyses of the extracted oil samples were performed for identification of the composition of organic molecules. Figure 12 shows the GC-MS spectra of the extracted oil samples from oil–quartz crystals and rock core samples. Unlike the oil samples extracted from rock core samples, The oil–quartz crystals showed several bands and later retention times indicated various molecules. Figure 13 and Table 2 show the ratio of the C number and the molecular list of oil compositions, respectively. Except the transition zone, the rate of C14 molecules was relatively high in extracted oils from oil–quartz crystals and rock core samples. In the transition zone, although a similar tendency was revealed in thin-sectional observation, it was the tendency that the extracted oil consisted of low-molecular weight molecules. The main components of organic molecules in the parent rock core and clay vein were aromatic molecules and alkanes, such as 2,4-di-tert-butylphenol, 1,3-di-tert-butylbenzene, and tridecane. Furthermore, aromatic esters and butyl phthalate were detected. Alkanes and alcohol molecules were primarily detected in the transition zone.
Figure 12.
GC-MS spectra of the extracted oil samples from rock core and oil–quartz crystal samples.
Figure 13.

Existence ratios of each carbon number molecule in the extracted oil samples from rock core and oil–quartz crystals samples. (a) Mother rock. (b) Transitional zone. (c) Vein clay. (d) Quartz.
Table 2. List of Detected Organic Molecules in Extracted Oil Samples from the Rock Core and Oil–Quartz Crystals Samples.
| chemical name | chemical formula | C number | classification | fluorescence | mother rock | transitional zone | vein clay | quartz–oil |
|---|---|---|---|---|---|---|---|---|
| 2,4-di-tert-butylphenol | C14H22O | 14 | aromatic | 28.247 | 25.279 | 16.502 | ||
| 1,3-di-tert-butylbenzene | C14H22 | 14 | aromatic | 17.532 | 8.491 | 17.472 | 10.530 | |
| p-tert-butylbenzoic acid | C11H14O2 | 11 | aromatic | 9.052 | ||||
| butyl phthalate | C16H22O4 | 16 | aromatic ester | 11.152 | ||||
| Kesscoflex MCP | C14H18O6 | 14 | aromatic ester | 9.434 | ||||
| tridecane | C13H28 | 13 | alkane | 4.221 | 5.204 | |||
| trans-2,2,4,5-tetramethyl-1,3-dioxolane | C7H14O2 | 7 | dioxolane | 3.896 | 16.038 | 3.346 | ||
| lycopene | C40H56 | 40 | terpene | + | 7.389 | |||
| 6-methyl-1-heptanol | C8H18O | 8 | alcohol | 6.034 | ||||
| 2-propyl-1-heptanol | C10H22O | 10 | alcohol | 4.126 | ||||
| 5-methyl-1-heptene | C8H16 | 8 | alkene | 3.633 | ||||
| myristic acid | C14H28O2 | 14 | fatty acid | 3.510 | ||||
| 3-methyl-5-undecene | C12H24 | 12 | alkene | 9.091 | ||||
| 6-methyl-1-octene | C9H18 | 9 | alkene | 2.278 | ||||
| 4-methyl-pent-4-en-2-one | C6H10O | 6 | diene | 3.247 | 13.208 | 3.717 | ||
| 4-methylheptane | C8H18 | 8 | alkane | 4.221 | 2.217 | |||
| 1,2-dimethylpropyl acetate | C7H14O2 | 7 | ketone | 3.247 | 12.264 | 3.346 | ||
| 2,3,4-trimethylhexane | C9H20 | 9 | alkane | 2.922 | ||||
| 1,2-diacetylethylene | C6H8O2 | 6 | alkane | 2.922 | 9.434 | |||
| 2,3,4-trimethylpentane | C8H18 | 8 | alkane | 4.461 | ||||
| 4,6-dimethyl-dodecane | C14H30 | 14 | alkane | 2.974 | ||||
| 5-hydroxy-2,4-di-tert-butylphenyl-pentansaeure | C19H30O3 | 19 | alcohol | 12.264 | ||||
| vinyl formate | C3H4O2 | 3 | aldehyde | 7.547 | ||||
| chloro-tert-butanol | C4H9ClO | 4 | alcohol | 5.660 | ||||
| 3,6,dimethyl-decane | C12H26 | 12 | alkane | 5.660 | ||||
| others | 20.455 | 23.048 | 34.729 | |||||
| 100 | 100 | 100 | 100 | |||||
In the case of oil–quartz crystals, although some of the detected molecules were the same as the cases of the parent rock core and clay vein parent, it was noteworthy that two C40 molecules were identified as terpene with fatty acid and low-molecular weight alcohol.
The detected terpene molecules were identified as lycopene and coleon F dimer. Noteworthily, lycopene and its derivatives exhibited fluorescence ability. Previous studies indicated that the irradiation band of lycopene was ∼415 nm.28−30 Then, we could conclude that one of the true characters of luminescence in oil–quartz crystals was lycopene at the least lower wavelength irradiation. On the other hand, a luminescence band of lycopene hardly coincided the irradiation band of 560 nm and fluorescence abilities of the detected molecules in the extracted oil samples. However, some previous studies indicated that the metal complexes of some aromatic molecules exhibited fluorescence ability, for example, lanthanoids.31−33 Then, we considered that the irradiation band of 560 nm originated such aromatic molecules with metal complex forms.
The typical skeleton crystal shapes of oil–quartz crystals and domains of oils indicated that oil was included quickly during the growth process of quartz crystals,34,35 and the quick growth of quartz crystals with oils caused the formation of the shapes. XRD measurements indicated that the clay vein parent contained few amounts of feldspar, whereas a large amount of feldspar was detected in the parent rock. Therefore, it was indicated that the feldspar decomposition process released the silica source for quick growth of quartz. Indeed, high temperature conditions (hydrothermal conditions) accelerated the decomposition process of feldspar and led to release of silica.36−38 However, organic molecules existing in oil–quartz crystals strongly regulated the upper temperature for growth fields. Considering the thermal stability of lycopene, the oil–quartz crystal formation reaction proceeded at ∼80 °C.39−41
The origin of oil in oil–quartz crystals was discussed. As shown in spectroscopic evaluations, the compositions of oil in oil–quartz crystals were different from those of rocks. The extracted oil from oil–quartz crystals contained terpene molecules (lycopene and coleon F), which were essential components of microorganism membranes.42,43 This observation suggested that the hydrothermal conditions, namely oil–quartz crystal formation field, might be also with bacterial coculture conditions and record the evidence of bacterial activity. Below 80 °C conditions, some thermophilic bacteria were likely to grow, such as Thermotoga Sp. and Pyrodictiaceae Sp..44−48 Thus, this foundation would introduce a novel interdisciplinary research field for geological events at lower temperature such as mineral formations affected by microorganisms.
3. Conclusions
We investigated the origin of oil–quartz crystals that exhibit fluorescence, obtained from Shimanto-cho, Kochi prefecture, Shikoku Island, Japan. The fluorescence domains in oil–quartz crystals were located at the voids of skeleton crystals. The origin of fluorescence was attributed to lycopene. Considering the thermal stability of lycopene and crystal morphology, oil–quartz crystals formed under extreme supersaturated conditions and low temperature (<80 °C).
4. Experimental Methods
4.1. Reagents for Analysis
All reagents were purchased from FUJI Film Wako Pure Chemical Inc. Co., Japan, as reagent grade.
4.2. Preparation of Bulk Analysis Samples of Rock Core and Quartz Samples
The obtained rock core samples were trimmed off the outer weathered part; then, the treated rock core samples and quartz samples were roughly crushed onto a steel anvil to prepare 1–2 cm-sized granules. The crushed granules were put into distilled water with a supersonic washing process three times. Then, the washed samples were dried in a dry oven at 40 °C for 2 d. The dried granules were well crushed using a preburned Al2O3 motor and pestles. Then, the crushed powder of the samples was stored in PP vessels.
4.3. Extraction of Oil Samples from Rock Core and Quartz Samples
Approximately 1 g of the prepared powder samples was soaked into 10 mL of reagent-grade acetone and tightly sealed. Then, the sealed samples were gently shaken at 60 rpm in a shaking incubator at 40 °C for 12 h. After soaking, the treated skim of the samples was filtrated using a 220 nm syringe filter. Then, the filtered skim of the samples was put into a PP tube; then, vacuum conditions were used to vaporize acetone.
4.4. Characterization of Solid-State Materials
All samples and location photographs were obtained using a digital camera (XP140, FUJI Film Co., Japan) at effective pixels of 16 Mpixels.
The crystallographic details of the samples were obtained using XRD (MiniFlex600, Rigaku Co., Japan) at an acceleration voltage and amplitude of 40 kV and 15 mA, respectively. The diffraction angle was continuously scanned over 2θ = 3–90° at a scanning rate of 2°/min for characterization.
The chemical bond structure of the samples was determined using Fourier transform infrared spectroscopy (FT-IR: IRTracer-100, Simadzu Co., Japan) equipped with a triglycine sulfate detector (20 scans, resolution 2 cm–1) with an attenuated total reflection diamond prism. All measurements were performed against an atmospheric background.
The atomic content of the samples was measured via energy-dispersive X-ray fluorescence spectroscopy (XRF: EDX-8100, Shimadzu Co., Japan) at an acceleration voltage of 15 kV under vacuum conditions.
The amount of CHN in the specimens was measured using carbon–hydrogen–nitrogen analysis (CHN-analysis: MT-6,YanacoCo., Kyoto, Japan) using Ar gas as the carrier gas after drying in a P2O5 desiccator. For organic C contents of sample evaluation, 0.1 g of the crushed rock sample was immersed into 15 mL of 25 wt % NaClO solution for 1 day at R.T. Then, the treated samples were washed with distilled water several times and eventually dried.
The fine structure of the samples was observed using field emission scanning electron microscopy (FE–SEM: JSM-6700F, JEOL Co., Japan) at an acceleration voltage of 3 kV after Os sputtering.
The 3D image of the samples was measured using a quantitative three-dimensional evaluation program, which is included in the microcomputed tomography system (microCT: ScanXmate-L080T, Comscantecno Co., Japan) with a source voltage (69 kV) and source current (149 μA) with an Al filter (0.5 mm). The voxel resolution was 125 μm3.
Gas chromatography–mass spectrometry (GC-MS) analyses were performed with a GCMS-QP2010 (Shimadzu Co., Japan) gas chromatograph magnetic sector mass spectrometer using He gas as a carrier gas with a constant flow of 1 mL/min. An Agilent J&W DB-5 (Agilent Technology Japan, Japan) capillary column (0.25 mm i.e., film thickness of 1.00 μm) with a 30 m-long integrated guard column was used. The GC oven was programmed for an initial temperature of 50 °C for 3 min, followed by heating at 10 °C/min to 250 °C with 37 min final hold time.
The fluorescence spectra of the samples were measured using a spectrofluorometer (FP-8550: JASCO Co., Japan) using irradiation wavelength at 265 nm and the measurement wavelength range from 275 to 850 nm at a scanning rate of 200 nm/min. Oil samples were coated onto the quartz glass for measurement.
Acknowledgments
The authors thank Dr. M. Kawasaki and A. Tokumoto for scientific discussions. The authors thank T. Baba (JASCO Co.) for fluorescence spectrometry measurements and Prof. H. Terasaki (Okayama Univ.) for microscopy observation. The authors thank Drs. Y. Suezawa, T. Nakanishi, and S. Asahi (RIST Kagawa) for helping with FT-IR and GC-MS measurements. This study was partially supported by the Research Center for Industrial Science & Technology, Kagawa Industry Support Foundation (RIST Kagawa). This study was financially supported by the Kazuchika Okura Memorial Foundation and JST Core Research for Evolutionary Science and Technology (JST-CREST), Grant number: JPMJCR22L5.
The authors declare no competing financial interest.
References
- Pearson D. G.; Brenker F. E.; Nestola F.; McNeill J.; Nasdala L.; Hutchison M. T.; Matveev S.; Mather K.; Silversmit G.; Schmitz S.; Vekemans B.; Vincze L. Hydrous mantle transition zone indicated by ringwoodite included within diamond. Nature 2014, 507, 221–229. 10.1038/nature13080. [DOI] [PubMed] [Google Scholar]
- Frezzotti M. L.; Tecce F.; Casagli A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. 10.1016/j.gexplo.2011.09.009. [DOI] [Google Scholar]
- Sobolev N. V.; Kaminsky F. V.; Griffin W. L.; Yefimova E. S.; Win T. T.; Ryan C. G.; Botkunov A. I. Mineral inclusions in diamonds from the Sputnik kimberlite pipe, Yakutia. Lithos 1997, 39, 135–157. 10.1016/S0024-4937(96)00022-9. [DOI] [Google Scholar]
- Promwongnan S.; Sutthirat C. Mineral Inclusions in Ruby and Sapphire from the Bo Welu gem Deposit in Chanthaburi, Thailand. Gem Gemol. 2019, 55, 354–369. 10.5741/GEMS.55.3.354. [DOI] [Google Scholar]
- Bui T. N.; Entremont P.; Gauthier J.-P. Large 12-Rayed Black Star Sapphire from Sri Lanka with Asterism Caused by Ilmenite Inclusions. J. Gemmol. 2017, 35, 430–435. 10.15506/JoG.2017.35.5.430. [DOI] [Google Scholar]
- Farfan G. A.; Post J. E. Quartz from Madagascar with Fuchsite Phantom Inclusions. J. Gemmol. 2019, 36, 698–699. 10.15506/JoG.2019.36.8.698. [DOI] [Google Scholar]
- Gurov V. V.; Tsvetkov E. G.; Kirdyashkin A. G. Features of beryllium aluminate crystal growth by the method of horizontally oriented crystallization. J. Cryst. Growth 2003, 256, 361–367. 10.1016/S0022-0248(03)01409-X. [DOI] [Google Scholar]
- VanSpeybroeck V.; Hemelsoet K.; Joos L.; Waroquier M.; Bell R. G.; Catlow C. R. A. Advances in theory and their application within the field of zeolite chemistry. Chem. Soc. Rev. 2015, 44, 7044–7111. 10.1039/C5CS00029G. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Liu N.; Lei Z.; Chen B. Selective Transformation of Various Nitrogen-Containing Exhaust Gases toward N2 over Zeolite Catalysts. Chem. Rev. 2016, 116, 3658–3721. 10.1021/acs.chemrev.5b00474. [DOI] [PubMed] [Google Scholar]
- Ye Q.; Zhou F.; Liu W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. 10.1039/c1cs15026j. [DOI] [PubMed] [Google Scholar]
- Mousa M.; Evans N. D.; Oreffo R. O. C.; Dawson J. I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. 10.1016/j.biomaterials.2017.12.024. [DOI] [PubMed] [Google Scholar]
- Nakamura R.; Takashima T.; Kato S.; Takai K.; Yamamoto M.; Hashimoto K. Electrical Current Generation across a Black Smoker Chimney. Angew. Chem., Int. Ed. 2010, 49, 7692–7694. 10.1002/anie.201003311. [DOI] [PubMed] [Google Scholar]
- Furukawa Y.; Sekine T.; Oba M.; Kakegawa T.; Nakazawa H. Biomolecule formation by oceanic impacts on early Earth. Nat. Geosci. 2009, 2, 62–66. 10.1038/ngeo383. [DOI] [Google Scholar]
- Kim H. J.; Furukawa Y.; Kakegawa T.; Bita A.; Scorei R.; Benner S. A. Evaporite borate-containing mineral ensembles make phosphate available and regiospecifically phosphorylate ribonucleosides: borate as a multifaceted problem solver in prebiotic chemistry. Angew. Chem., Int. Ed. 2016, 55, 15816–15820. 10.1002/anie.201608001. [DOI] [PubMed] [Google Scholar]
- Momma K.; Ikeda T.; Nishikubo K.; Takahashi N.; Honma C.; Takada M.; Furukawa Y.; Nagase T.; Kudoh Y. New silica clathrate minerals that are isostructural with natural gas hydrates. Nat. Commun. 2011, 2, 196–202. 10.1038/ncomms1196. [DOI] [PubMed] [Google Scholar]
- Nakamura T.; Matsumoto M.; Amano K.; Enokido Y.; et al. Formation and evolution of carbonaceous asteroid Ryugu: Direct evidence from returned samples. Science 2022, 379, eabn8671 10.1126/science.abn8671. [DOI] [PubMed] [Google Scholar]
- Parnell J.; Carey P. F.; Monson B. Fluid inclusion constraints on temperatures of petroleum migration from authigenic quartz in bitumen veins. Chem. Geol. 1996, 129, 217–226. 10.1016/0009-2541(95)00141-7. [DOI] [Google Scholar]
- Munz I. A.; Yardley B. W. D.; Banks D. A.; Wayne D. Deep penetration of sedimentary fluids in basement rocks from southern Norway: Evidence from hydrocarbon and brine inclusions in quartz veins. Geochim. Cosmochim. Acta 1995, 59, 239–254. 10.1016/0016-7037(94)00322-D. [DOI] [Google Scholar]
- Feely M.; Costanzo A.; Lindner F.; George J.; Parnell J.; Bowden S.; Baba M.; Owens P. Quartz-Amethyst Hosted Hydrocarbon-Bearing Fluid Inclusions from the Green Ridge Breccia in the Snoqualmie Granite, North Cascades,WA, USA. Minerals 2017, 7, 174 10.3390/min7090174. [DOI] [Google Scholar]
- George S. C.; Volk H.; Dutkiewicz A.; Ridley J.; Buick R. Preservation of hydrocarbons and biomarkers in oil trapped inside fluid inclusions for >2 billion years. Geochim. Cosmochim. Acta 2008, 72, 844–870. 10.1016/j.gca.2007.11.021. [DOI] [Google Scholar]
- Suchý V.; Dobes P.; Sykorova I.; Machovic V.; Stejskal M.; Kroufek J.; Chudoba J.; Matejovsky L.; Havelcova M.; Matysova P. Oil-bearing inclusions in vein quartz and calcite and, bitumens in veins: Testament to multiple phases of hydrocarbon migration in the Barrandian basin (lower Palaeozoic), Czech Republic. Mar. Pet. Geol. 2010, 27, 285–297. 10.1016/j.marpetgeo.2009.08.017. [DOI] [Google Scholar]
- Katto J. Sedimentary structures from the Shimanto Terrain, Shikoku, Southwest Japan. Res. Rep. Kochi Univ. 1961, 13, 45–58. [Google Scholar]
- Geological map: Kubokawa, Kanehara N. eds., Imperial Geological Survey of Japan, 1933.
- Taira A.Sedimentary evolution of Shikoku subduction zone: The Shimanto Belt and Nankai Trough. In Formation of Active Ocean Margins; Nasu N.; Kobayashi K.; Uyeda S.; Kushiro I.; Kagami H., Eds.; Terrapub, 1985; pp 835–851. [Google Scholar]
- Yanai S. Megakink bands and Miocene regional stress field in Outer Southwest Japan. Sci. Pap. Coll. Arts. Sci. Univ. Tokyo 1986, 36, 55–79. [Google Scholar]
- Kiminami K.; Oyaizu A.; Ishihama S.; Miura K. Chemical composition of sandstones from the Cretaceous Shimanto Belt, western Shikoku, Japan, and correlation of petrofacies units in the Northern Shimanto Belt. Mem. Geol. Soc. Jpn. 2000, 57, 107–117. [Google Scholar]
- Oyaizu A.; Miura K.; Tanaka T.; Hayashi H.; Kiminami K. Geology and radiolarian ages of the Shimanto Supergroup, western Shikoku, Southwest Japan. J. Geol. Soc. Jpn. 2002, 108, 701–720. 10.5575/geosoc.108.11_701. [DOI] [Google Scholar]
- Alwis D. D. D. H.; Chandrika U. G.; Jayaweera P. M. Spectroscopic studies of neutral and chemically oxidized species of β-carotene, lycopene and norbixin in CH2Cl2: Fluorescence from intermediate compounds. J. Lumin. 2015, 158, 60–64. 10.1016/j.jlumin.2014.08.036. [DOI] [Google Scholar]
- Numan N.; Jeyaram S.; Kaviyarasu K.; Neethling P.; Sackey J.; Kotsedi C. L.; Akbari M.; Morad R.; Mthunzi-Kufa P.; Sahraoui B.; Maaza M. On the remarkable nonlinear optical properties of natural tomato lycopene. Sci. Rep. 2022, 12, 9078 10.1038/s41598-022-12196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii R.; Onaka K.; Nagae H.; Koyamaa Y.; Watanabe Y. Fluorescence spectroscopy of all-trans-lycopene: comparison of the energy and the potential displacements of its 2Ag- state with those of neurosporene and spheroidene. J. Lumin. 2001, 92, 213–222. 10.1016/S0022-2313(00)00260-X. [DOI] [Google Scholar]
- Tian D.; Li Y.; Chen R.-Y.; Chang Z.; Wang G.-Y.; Bu X.-H. A luminescent metal–organic framework demonstrating ideal detection ability for nitroaromatic explosives. J. Mater. Chem. A 2014, 2, 1465–1470. 10.1039/C3TA13983B. [DOI] [Google Scholar]
- Bradbury A. J.; Lincoln S. F.; Wainwright K. P. Fluorescence signaling of aromatic oxoanion inclusion within metal-ion activated molecular receptor complexes formed from 2-(9-anthracenylmethylamino)ethyl-appended cyclen. J. Inclusion Phenom. Macrocyclic Chem. 2011, 71, 567–575. 10.1007/s10847-011-9924-7. [DOI] [Google Scholar]
- Marmodée B.; de Klerk J. S.; Ariese F.; Gooijer C.; Kumke M. U. High-resolution steady-state and time-resolved luminescence studies on the complexes of Eu(III) with aromatic or aliphatic carboxylic acids. Anal. Chem. Acta 2009, 652, 285–294. 10.1016/j.aca.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Barbee O.; Chesner C.; Deering C. Quartz crystals in Toba rhyolites show textures symptomatic of rapid crystallization. Am. Mineral. 2020, 105, 194–226. 10.2138/am-2020-6947. [DOI] [Google Scholar]
- Sunagawa I.Crystals Growth, Morphology and Perfection, Cambridge University Press: U.K., 2005. [Google Scholar]
- Su S.; Ma H.; Chuan X. Hydrothermal decomposition of K-feldspar in KOH–NaOH–H2O medium. Hydrometallurgy 2015, 156, 47–52. 10.1016/j.hydromet.2015.05.014. [DOI] [Google Scholar]
- Schepers A.; Milsch H. Dissolution–precipitation reactions in hydrothermal experiments with quartz–feldspar aggregates. Contrib. Mineral. Petrol. 2013, 165, 83–101. 10.1007/s00410-012-0793-x. [DOI] [Google Scholar]
- Wild B.; Daval D.; Guyot F.; Knauss K. G.; Pollet-Villard M.; Imfeld G. pH-dependent control of feldspar dissolution rate by altered surface layers. Chem. Geol. 2016, 442, 148–159. 10.1016/j.chemgeo.2016.08.035. [DOI] [Google Scholar]
- Hackett M. M.; Lee J. H.; Francis D.; Schwartz S. J. Thermal Stability and Isomerization of Lycopene in Tomato Oleoresins from Different Varieties. J. Food Sci. 2006, 69, 536–541. 10.1111/j.1365-2621.2004.tb13647.x. [DOI] [Google Scholar]
- Qiu W.; Jiang H.; Wang H.; Gao Y. Effect of high hydrostatic pressure on lycopene stability. Food Chem. 2006, 97, 516–523. 10.1016/j.foodchem.2005.05.032. [DOI] [Google Scholar]
- Chen J.; Shi J.; Xue S. J.; Ma Y. Comparison of lycopene stability in water- and oil-based food model systems under thermal- and light-irradiation treatments. LWT – Food Sci. Technol. 2009, 42, 740–747. 10.1016/j.lwt.2008.10.002. [DOI] [Google Scholar]
- Hayakawa H.; Motoyama K.; Sobue F.; Hemmi H.; et al. Modified mevalonate pathway of the archaeon Aeropyrum pernix proceeds via trans-anhydromevalonate 5-phosphate. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 10034–10039. 10.1073/pnas.1809154115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeno D.; Tobias A. V.; Arnold F. H. Evolution of the C30 Carotenoid Synthase CrtM for Function in a C40 Pathway. J. Bacteriol. 2002, 184, 6690–6699. 10.1128/JB.184.23.6690-6699.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashefi K.; Lovely D. R. Extending the Upper Temperature Limit for Life. Science 2003, 301, 934 10.1126/science.1086823. [DOI] [PubMed] [Google Scholar]
- Brock T. D.; Freeze H. Thermus aquaticus gen. n. and sp. n., a Nonsporulating Extreme Thermophile. J. Bacteriol. 1969, 98, 289–297. 10.1128/jb.98.1.289-297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetter K. O. Ultrathin mycelia-forming organisms from submarine volcanic areas having an optimum growth temperature of 105 °C. Nature 1982, 300, 258–260. 10.1038/300258a0. [DOI] [Google Scholar]
- Stetter K. O.; König H.; Stackebrandt E. Pyrodictium gen. nov., a new genus of submarine disc-shaped sulphur reducing archaebacteria growing optimally at 105 °C. Syst. Appl. Microbiol. 1983, 4, 535–551. 10.1016/S0723-2020(83)80011-3. [DOI] [PubMed] [Google Scholar]
- Huber R.; Langworthy T. A.; König H.; Thomm M.; Woese C. R.; Sleytr U. B.; Stetter K. O. Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90 °C. Arch. Microbiol. 1986, 144, 324–333. 10.1007/BF00409880. [DOI] [Google Scholar]
- Levien L.; Prewitt C. T.; Weidner D. J. Structure and elastic properties of quartz at pressure P = 1 atm. Am. Mineral. 1980, 65, 920–930. [Google Scholar]
- Grundy H. D.; Ito J. The refinement of the crystal structure of a synthetic non-stoichiometric Sr feldspar. Am. Mineral. 1974, 59, 1319–1326. [Google Scholar]
- Viani A.; Gualtieri A.; Artioli G. The nature of disorder in montmorillonite by simulation of X-ray powder patterns. Am. Mineral. 2002, 87, 966–975. 10.2138/am-2002-0720. [DOI] [Google Scholar]