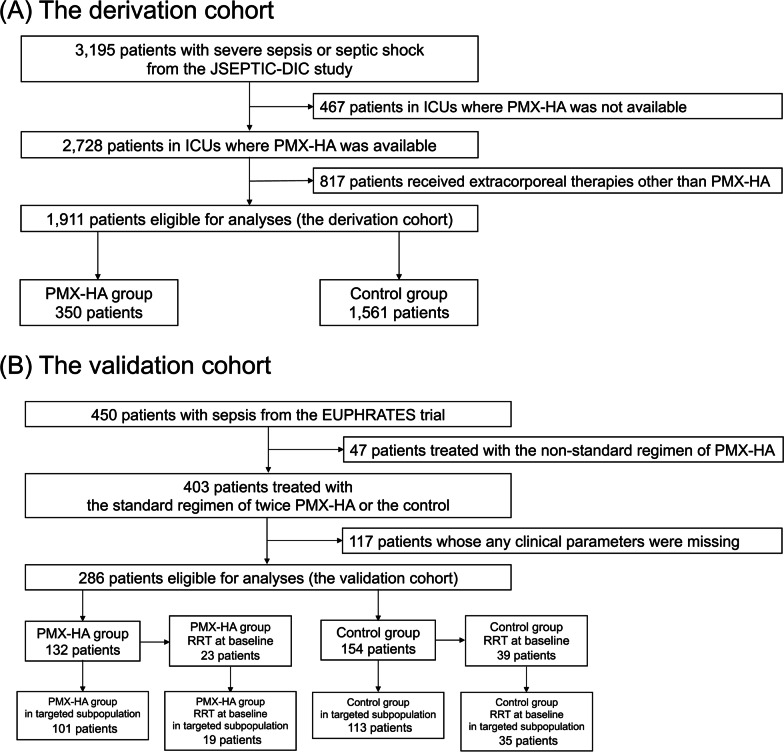
Fig. 1.
Flow diagram of patients eligible for analysis in the derivation and validation cohorts. Legend: A Among 3195 patients with severe sepsis or septic shock based on the International Sepsis Definitions Conference criteria from the JSEPTIC-DIC study, 2011–2013, we identified 1911 patients with sepsis who did not receive extracorporeal therapies other than PMX-HA as the derivation cohort. B Among 450 patients with septic shock from the EUPHRATES trial, 2010–2016, we identified 286 patients who received the standard regimen of PMX-HA treatments or standard of care without PMX-HA who had complete data available (i.e., APACHE II score, SOFA score, WBC count, platelet count, PT-INR, and lactate). PMX-HA = Polymyxin B Hemadsorption, RRT = Renal Replacement Therapy, APACHE II score = Acute Physiology and Chronic Health Enquiry II score, SOFA score = Sequential Organ Failure Assessment score, WBC = White Blood Cell, PT-INR = Prothrombin Time and International Normalized Ratio